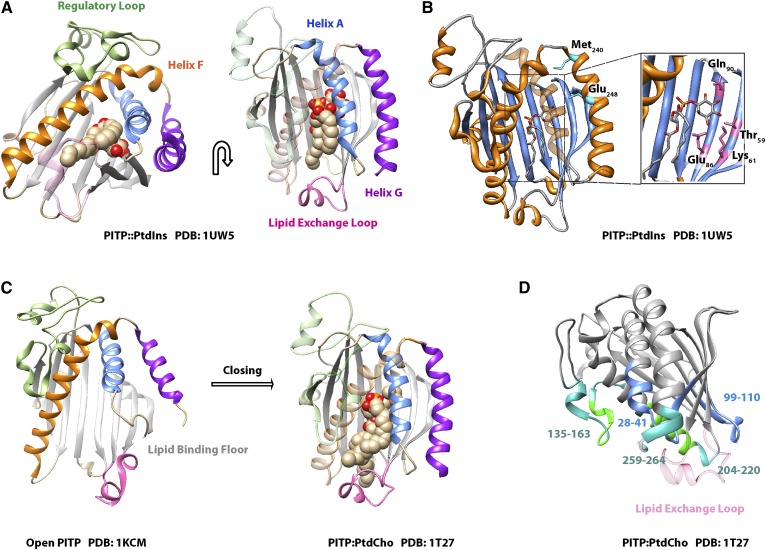
Fig. 7.
START PITP structure. A: The crystal structure of PITPα bound to PtdIns is shown in two orientations as indicated by the arrow. The β-sheet floor of the lipid binding pocket (gray), the regulatory loop (green), helix F (orange), helix A (blue), helix G (purple), and the lipid exchange loop (pink) are highlighted. B: The PtdIns headgroup-binding motif of PITPα is illustrated and the coordinating residues within the lipid binding pocket (inset: Thr59, Lys61, Glu86, Gln90) are highlighted. Residues on helix G that do not bind the headgroup directly but modulate the conformational dynamics that specifically influence PtdIns-binding are also highlighted (cyan: Met240, Glu248). C: The crystal structures of open lipid-free PITPα and PtdCho-bound PITPα are shown to illustrate the conformational dynamics associated with gating of the hydrophobic lipid-binding pocket during the exchange cycle. The lipid exchange loop controls access to the pocket and helix G and helix A fold over the lipid binding floor. Coloring scheme is as in A. D: The crystal structure of PITPα bound to PtdCho is shown with membrane-association regions. Hydrophobic interactions with the membrane are rendered in green and involve residues 135-163 and 259-264, while electrostatic interactions with the membrane involve residues 28-41 and 99-110 and are highlighted in blue. Residues that interact with the membrane only when PtdIns is present in the bilayer are indicated in bright green. The lipid exchange loop is made translucent for the purpose of clarity, and this substructure penetrates the bilayer surface.