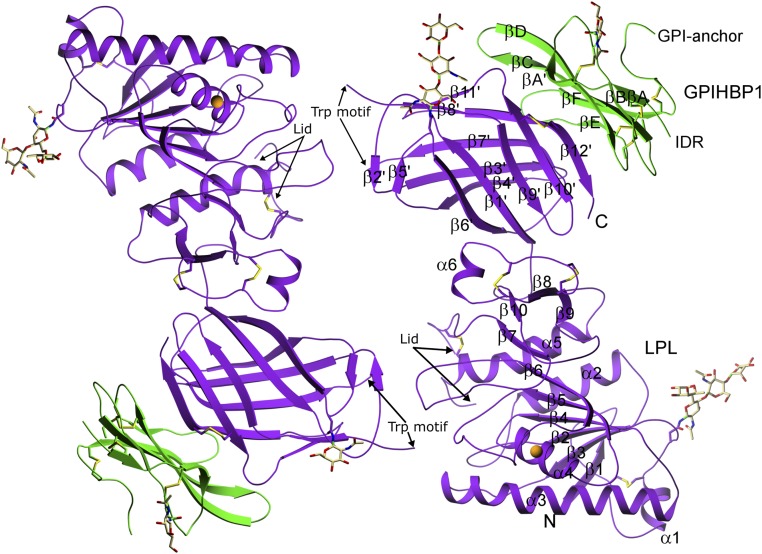
Fig. 2.
Structure of the LPL–GPIHBP1 complex, as depicted by ribbon representations of the two LPL–GPIHBP1 complexes in the asymmetric crystallographic unit. LPL (purple) has five disulfide bonds (C54–C67, C243–C266, C291–C302, C305–C310, and C445–C465); a single calcium ion (orange sphere) coordinated by A194, R197, S199, D201, and D202; and two N-linked glycans (at N70 and N386). GPIHBP1 (green) has one N-linked glycan (at N78). LPL contains an N-terminal α/β-hydrolase domain (N) containing 6 α-helices and 10 β-strands and a C-terminal flattened β-barrel domain (C) containing 12 β-strands, connected by a hinge region. The numbering of the β-strands in GPIHBP1 follows the nomenclature proposed for LU domain proteins (55). IDR, intrinsically disordered region.