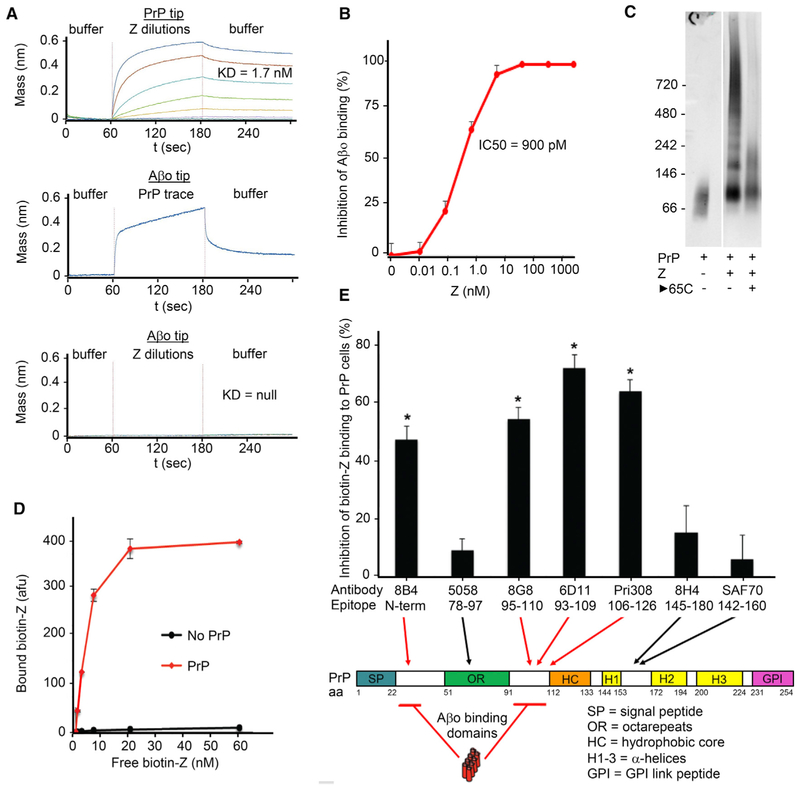
Figure 2. Purified Compound Z Binds PrPC Reversibly, Inhibiting Aβo/PrPC Interaction via Competition for PrPC Aβo-Binding Domains.
(A) Biolayer interferometric association (60–180 s) and dissociation (180–300 s) traces of 10–20 kDa Z with PrPC-coated sensor in 4-fold dilution steps from 1 μM top concentration, indicating a dissociation constant of 1.7 nM (top graph). Aβo-coated sensor detects soluble full-length PrPC interaction but not compound Z (middle and bottom graphs, respectively).
(B) PLISA measurement of 10–20 kDa Z Aβo/PrPC inhibitory activity, indicating an IC50 of 910 pM. Data are mean ± SEM, n = 3 replicates per sample.
(C) PrPC immunoblot of non-denaturing gel-shift assay of full-length PrPC incubated with 10–40 kDa Z. Laddering indicates multiple PrPC molecules bound per Z molecule. Incubation at 65°C after co-incubation shows reversible association of Z and PrPC.
(D) Biotinylated 10–20 kDa Z binds full-length PrPC-coated plate concentration-dependently. Data are mean ± SEM, n = 3 replicates per sample.
(E) Binding of biotinylated Z to PrPC-coated plate is inhibited by antibodies directed against either of the two Aβo-binding domains on PrPC and not by antibodies against other regions of PrP, indicating direct and selective Z interaction with PrPC Aβo-binding domains. Data are mean ± SEM, n = 3 replicates per sample. *p <0.05, Student’s t test.