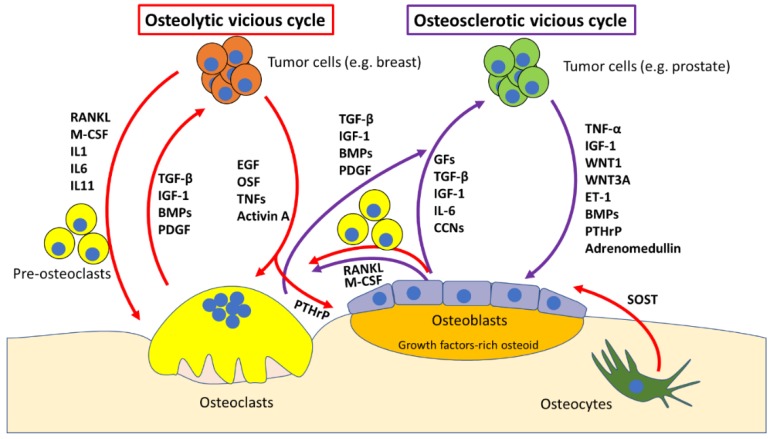
Figure 1.
Cartoon representing the osteolytic and osteosclerotic vicious cycles. Osteolytic cycle: osteolysis-generating cancer cells (e.g., breast) migrate to the bone marrow and start secreting osteoclast-stimulating factors such as epithelial growth factor (EGF), osteoclast stimulating factor (OSF), tumour necrosis factors (TNFs) and Activin A. In parallel, cancer cells also secrete osteoclast-differentiating factors, such as receptor activator of nuclear factor kB ligand (RANKL), macrophage-colony stimulating factor (M-CSF) and Interleukin (IL)-1, 6 and 11, which promote differentiation of pre-osteoclasts into osteoclasts. The bone matrix is rich in growth factors (GFs), transforming growth factor (TGF)-β, insulin-like growth factor (IGF)-1, bone morphogenic proteins (BMPs) and platelet-derived growth factor (PDGF). These factors get released when osteoclasts resorb bone, and they promote tumour growth, which closes the osteolytic vicious cycle. Osteocytes also take a crucial part in the process, secreting sclerostin (SOST) in response to osteolytic cancers (especially multiple myeloma), which inhibits osteoblast activity and the Wnt- β-catenin pathway Osteosclerotic cycle: osteosclerosis-generating cancer cells (e.g., prostate) migrate to the bone marrow and start secreting osteoblast-stimulating factors such as TNF-α, IGF-1, Wingless-type MMTV integration site (WNT)1, WNT3A, Endothelin (ET)-1, BMPs, parathyroid hormone-related peptide (PTHrP) and adrenomedullin. This stimulates osteoblast differentiation and activity. On one hand this leads to osteoblast-mediated osteoclastogenesis by increasing osteoblastic expression of RANKL and M-CSF, which causes the release of growth factors as discussed for the osteolytic cycle. On the other hand, osteoblasts themselves release a plethora of factors including GFs, TGF-β, IGF-1, IL-6 and chemokines (CCNs), which stimulate tumour growth, closing the osteosclerotic (which most of the times has an osteolytic component as well) vicious cycle.