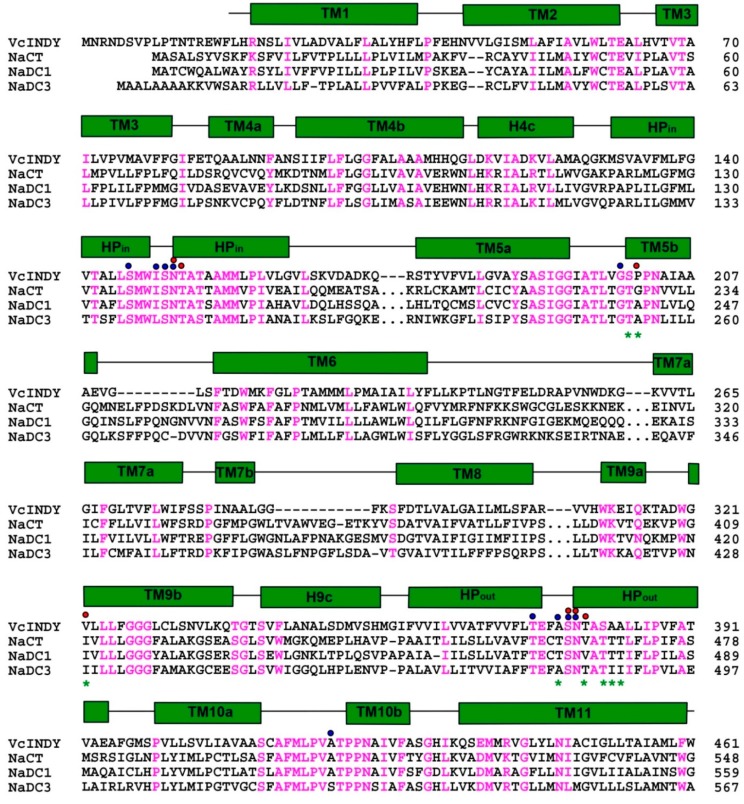
Figure 2.
Amino-acid sequence alignment of representative DASS proteins. Residues conserved among VcINDY and three human orthologues are colored magenta, regions of secondary structural elements in VcINDY are outlined, with transmembrane helices shown as green rectangles. Red and blue dots highlight amino acids that bind succinate/citrate and Na+, respectively. Positions for the relevant humanizing mutations, which include S200T, P201G, V322I, T379V, A376T, S381T, A382T, and A383T, are marked by green asterisks. For clarity, some residues in the human DASS proteins were omitted and indicated by “…”. Notably, the amino-acid sequence identity between VcINDY and NaCT is 23%, but the degree of sequence conservation in and around the citrate- and Na+-binding sites is substantially higher, suggesting that the VcINDY structure provides a useful model for studying the mechanism of NaCT or other human DASS. Sequence alignment was performed by using the program ClustalW.