Figure 7.
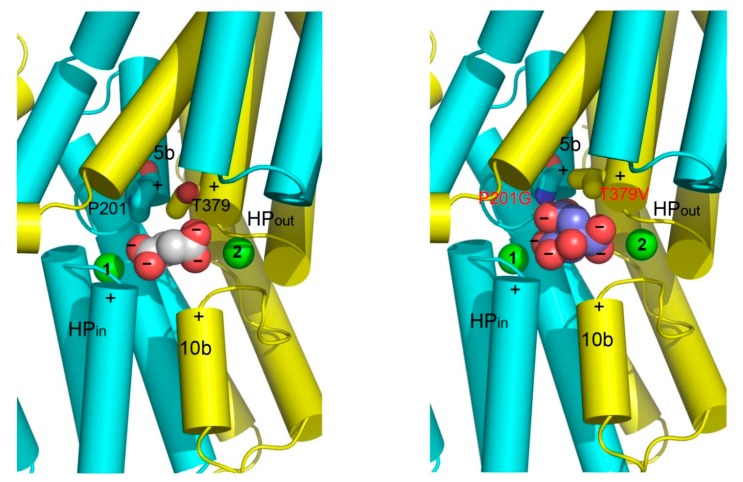
Structural basis for substrate recognition by DASS. The N and C domains in VcINDY (left panel) and its humanized variant (right panel) are colored cyan and yellow, respectively. Relevant amino acids are drawn as stick models, whereas the bound succinate (left panel) or citrate (right panel), as well as the Na+ ions (green) are shown as spheres. Positive helix dipoles for short helices TM5b and TM10b are highlighted by plus signs, whereas the negatively charged carboxylates in succinate or citrate are indicated by minus signs. The charged state of succinate or citrate is deduced based on the crystallization pH (~7). Both the helix dipoles and Na+ appear to contribute to the anion binding. Furthermore, P201 and T379 may enable VcINDY to select for succinate but against citrate. In MT5, P201, and T379 are replaced by Gly and Val, respectively, which may allow the membrane-embedded transporter to bind citrate more strongly than VcINDY.