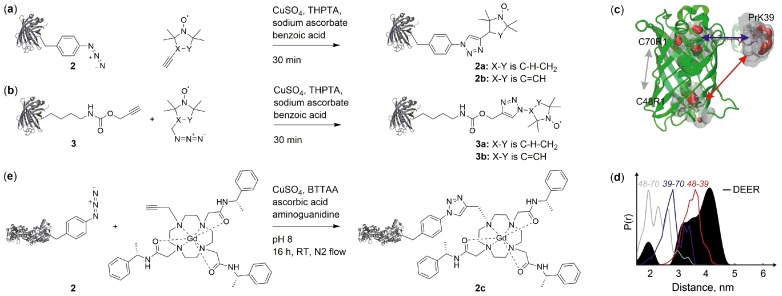
Figure 4.
SDSL by copper catalyzed azide-alkyne cycloadditions (CuAAC). (a) CuAAC between ncAA 2 with azide function and a proxyl- or pyrrolin-based nitroxide label, resulting in 2a and 2b [47]. (b) CuAAC with ncAA 3 bearing an alkyne function and proxyl- or pyrroline-based azides, resulting in 3a or 3b [47]. (c) Nitroxide incorporation positions for DEER measurements with eGFP. Labels were introduced at Y39 via CuAAC using ncAA 3 (“PrK”) and at naturally occurring cysteines C48 and C70 via methanethiosulfonate spin label (MTSL) (PDB ID 4EUL [48]). (d) Comparison of DEER data with rotamer-based model reveals accessibility of the cysteines [47]. Measured distance distribution of eGFP Y39 3a, C70 R1 and C48 R1 (black) compared to modeled distance distributions for the respective doubly labeled protein (grey, blue, and red). (c+d) Reprinted from Orthogonal spin labeling using click chemistry for in vitro and in vivo applications, J. Magn. Res. 2017 275, 38–45 [47], Copyright (2017), with permission from Elsevier. (e) CuAAC between ncAA 2 with azide function and a Gd(III) label [41,42] resulting in 2c. Either tris-[(1-hydroxy-propyl-1H-1,2,3-triazol-4-yl)methyl]amine (THPTA, a+b) or 2-[4-((bis[(1-tert-butyl-1H-1,2,3-triazol-4-yl)methyl]-amino)methyl)-1H-1,2,3-triazol-1-yl]acetic acid (BTTAA, e) was used as copper-complexing ligand.