Abstract
When plants are exposed to drastic environmental changes such as drought, salt or bacterial invasion, rapid stomatal movement confers tolerance to these stresses. This process involves a variety of guard cell expressed ion channels and their complex regulation network. Inward K+ channels mainly function in stomatal opening. On the other hand, guard cell anion channels play a crucial role in the closing of stomata, which is vital in terms of preventing water loss and bacterial entrance. Massive progress has been made on the research of these anion channels in the last decade. In this review, we focus on the function and regulation of Arabidopsis guard cell anion channels. Starting from SLAC1, a main contributor of stomatal closure, members of SLAHs (SLAC1 homologues), AtNRTs (Nitrate transporters), AtALMTs (Aluminum-activated malate transporters), ABC transporters, AtCLCs (Chloride channels), DTXs (Detoxification efflux carriers), SULTRs (Sulfate transporters), and their regulator components are reviewed. These membrane transport systems are the keys to maintaining cellular ion homeostasis against fluctuating external circumstances.
Keywords: guard cell, drought stress, salt stress, bacterial immunity, anion channel, protein kinases, calcium signaling, abscisic acid signaling, ion homeostasis
1. Introduction
When exposed to saline or water-deprived condition, plants respond in various ways to increase their survival rate. Stomatal movement is one of the key features of this response. Whenever plant senses salt or drought stress, and the consequent demand for saving water, rapid stomatal closure is induced to prevent water loss. In addition, pathogen attack can also trigger stomatal closure, for stomata can be a pathway for bacterial infestation. It is well known that light induced stomatal opening is controlled by inward-rectifying K+ channels localized in guard cell PM (plasma membrane), such as KAT1, KAT2, AKT1, and AKT2 [1,2,3,4,5,6,7,8]. In contrast, stomatal closure consists of guard cell expressed outward K+ channels and anion channels. Particularly, when plants are exposed to the stressful conditions mentioned above, activation of guard cell anion channels holds the key to defending themselves by inducing rapid stomatal closure.
Plant cells contain several types of anions including chloride, nitrate, sulfate, and organic acids like malate. Chloride is a major component of salt in soil, alongside with sodium, a major inflictor of salt damage to plants. While chloride acts as an essential nutrient, accumulation of chloride in the shoot (even without the presence of sodium) causes a decreased rate of transpiration and photosynthesis, leading to reduced crop yield and quality [9,10,11]. Nitrate works as an essential nitrogen source for amino acid synthesis. The process starting from direct uptake of nitrate from the soil, followed by enzymatic reactions and consequent production of glutamate, is an exclusive feature for plants (nitrogen assimilation) [12,13]. Nitrate also acts as an antagonist against chloride and could be applied to prevent Cl over-accumulation in shoots. Malate is important as an intermediate of TCA-cycle, essential storage carbon molecules and major photosynthate in CAM and C4 plants [14,15,16,17,18,19,20,21,22,23]. It also participates in the biosynthesis of amino acids and fatty acids, root growth, and aluminum tolerance [24,25,26]. Sulfate is an essential source for the biosynthesis of cysteine. Cysteine can either be directly incorporated into a protein or a peptide such as glutathione (GSH), or can be used as a sulfur donor for various coenzymes like molybdenum cofactor required for ABA synthesis [27,28,29,30].
These anions, once produced or uptaken, are immediately transferred to appropriate tissues and cell compartments by various anion transport systems, anion channels and transporters. The anion membrane transport system is distributed in each cell of the entire plant, and, in particular, many types of anion channels function intensively in guard cells, since the cell volume fluctuates in response to frequent environmental changes. The mechanism of stress-induced stomatal closure thus relies on these guard cell expressed anion channels. Under stress conditions, they drive anions either outside the cell or into the vacuole, triggering change in cell turgor pressure and consequently reducing the volume of guard cells. In this review, we focus on the function and regulation of such anion channels in guard cell.
2. SLAC1, a Major Contributor of Stomatal Closure
Early patch clamp studies in the 1980s revealed two types of anion channels present in guard cell PM: R (rapid)-type and S (slow)-type. R-type channel activated rapidly within 50 ms by depolarization, while S-type channel showed slow voltage-dependent activation and deactivation [31,32,33,34]. Then, 2008 saw a breakthrough: an ozone-sensitive Arabidopsis mutant named rcd3 (radical-induced cell death3) was isolated, showing constitutively higher stomatal conductance and deficit in the well-known activations of S-type guard cell anion channels by Ca2+ or abscisic acid (ABA) [31,32,35,36,37,38]. This mutant was renamed SLAC1 (slow anion channel-associated 1), and afterwards, SLAC1 gene was shown to encode a guard cell expressed S-type anion channel [38,39].
SLAC1 is predicted to be a membrane protein with ten transmembrane helices which, based on the structure of its bacterial homologue, forms a symmetrical trimer [38,40,41]. Usually in its inactive state, in which a phenylalanine residue at position 450 (Phe450) blocks its channel pore, SLAC1 is only activated when it is phosphorylated by certain kinases and a conformational change allows the removal of the Phe450 residue [40,42,43,44]. Various kinases are involved in this activation of SLAC1, including SnRK (sucrose non-fermenting-related kinase), LRR-RLK (leucine-rich repeat kinase), MPK (mitogen-activated protein kinase), CPK/CDPK (calcium dependent kinase), CBL (calcineurin-B like protein) and CIPK (CBL-interacting protein kinase) (Table 1; Figure 1) [45,46,47,48,49,50,51,52,53,54,55].
Table 1.
List of guard cell expressed ion channels/transporters and their regulator components.
| Name | Subcellular Localization | Function | Activation or Deactivation | Regulatory Components | Reference |
|---|---|---|---|---|---|
| SLAC1 | PM | Cl− efflux | A | OST1 | [56,57,61] |
| A | GHR1 | [64,81] | |||
| A | MPK9/12 | [54,67] | |||
| A | CPK3/6/21/23 | [61,82,83] | |||
| A | CBL1/9-CIPK23 | [83] | |||
| A | CBL5-CIPK11 | [55] | |||
| A | BAK1 (via OST1 phosphorylation) | [68,84,85] | |||
| A | BIK1 (via ROS production) | [86,87] | |||
| SLAH3 | PM | NO3− efflux | A | CPK3/6/21/23 | [83,88,89] |
| A | CBL1/9-CIPK23 | [83] | |||
| CHL1 | PM | NO3− influx | D | CBL1/9-CIPK23 (via conversion of nitrate transport mode) |
[90,91,92,93] |
| AtALMT4 | Tonoplast | Malate efflux/influx | [94] | ||
| AtALMT6 | A | Ca2+ | [95] | ||
| AtALMT9 | Tonoplast | Cl− influx | A | Cytosolic malate | [96] |
| AtALMT12 | PM | malate efflux | A | OST1 | [97] |
| AtABCB14 | PM | malate influx | [98] | ||
| AtCLCa | Tonoplast | H+ efflux/NO3− influx | A | CBL1/9-CIPK23 | [99,100,101] |
| AtCLCc | Tonoplast | [102] | |||
| DTX33 | Tonoplast | Cl− influx | [103,104,105] | ||
| DTX35 | |||||
| SULTR3;1 | Chloroplast | SO4− influx | [106] | ||
| KAT1 | PM | K+ influx | D | OST1 | [62,63] |
| D | SLAC1, SLAH3 | [107] | |||
| AKT1 | PM | K+ influx | D | CBL1/9-CIPK23 | [108,109,110] |
| GORK | PM | K+ efflux | A | CPK21 | [111] |
| NHX1 | Tonoplast | H+ efflux/K+ influx | A | CBL2/3, CIPK9/17 | [112] |
| NHX2 |
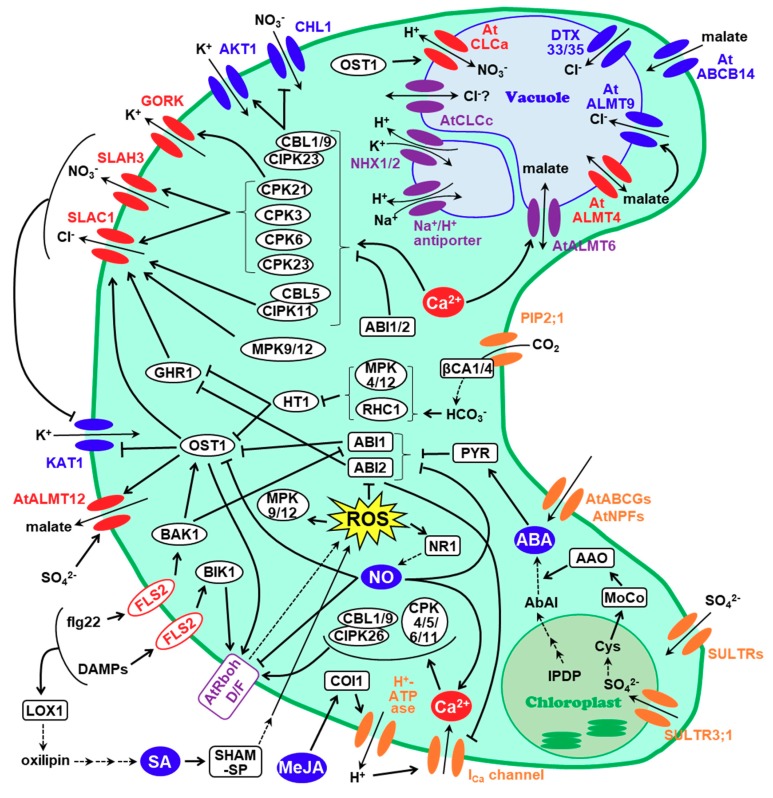
Figure 1.
Schematic model of ion channel regulation during stress induced stomatal movement in Arabidopsis thaliana. Thin arrow, influx/efflux of compounds; thick arrow, activation or inhibition; broken arrow, breakdown or biosynthesis. Channels/Transporters, which have been evidenced to control stomatal opening or closure, are shown in red and blue, respectively. Channels shown in purple are the ones that contribute to both opening and closure, or their role in stomatal regulation still remains ambiguous. Other channels/transporters are shown in orange. Abbreviations; SHAM-SP, SHAM-sensitive peroxidase; IPDP, isopentenyl diphosphate; AbAl, abscisic aldehyde; MoCo, molybdenum cofactor; AAO, abscisic aldehyde oxidase.
Drought/salt responsive stomatal closure occurs by drought-driven synthesis of ABA, which is an essential signaling hormone for some activators of SLAC1. SnRK2.6 also known as OST1 (Open stomata 1), identified through functional screening of Arabidopsis mutants, was the first reported kinase to activate SLAC1 [56,57]. Activity of OST1 is usually suppressed by the protein phosphatase ABI1. Application of ABA induces protein complex formation of ABI1 and ABA receptor proteins PYR/PYL/RCAR, which subsequently inactivates ABI1 and allows OST1 to phosphorylate Ser120 residue of SLAC1 [45,46,56,57,58,59,60,61]. OST1 also possesses the ability to phosphorylate and inactivate K+ in channel KAT1, further emphasizing its significance in the closing of stomata [62,63]. In addition to this, a member of LRR-RLK named GHR1 (guard cell hydrogen peroxide-resistant 1) was identified as an alternative key kinase in ABA-dependent regulation of SLAC1 activity. In contrast to OST1, GHR1 interacts with protein phosphatase ABI2 and not ABI1, suggesting that GHR1 acts in parallel with OST1 upon ABA-induced stomatal closure [64]. OST1, activated by ABA, is also capable of promoting ROS (reactive oxygen species) production in guard cell by phosphorylating NADPH oxidase RbohD and RbohF [63,65,66]. Two MPKs, MPK9 and MPK12, are known to mediate ROS-induced S-type channel activation in guard cell [54,67]. Murata et al. reported that ABI2, not ABI1, was inhibited by ROS, suggesting a ROS-mediated indirect enhancement of GHR1 activity [51,58,64]. In a recent study, another kinase BAK1 (Brassinosteroid insensitive 1-associated receptor kinase 1) was shown to directly form a complex with OST1 and stimulate stomatal closure in an ABA-dependent manner. BAK1 functions upstream of ROS production, and its complex formation with OST1 is inhibited by ABI1 [68]. In addition, accumulation of ROS (particularly H2O2) enhances synthesis of nitric oxide (NO) by NR1 (nitrate reductase 1) [69,70]. NO can provide both positive or negative feedback in stomatal closure [70,71,72,73]. NO induces phosphatidic acid (PA) production via activation of phospholipase C or D, which in turn inhibits ABI1 and activates RbohD/RbohF [74,75,76,77]. On the other hand, accumulation of NO triggers degradation of ABA receptors PYR/PYL/RCAR via tyrosine nitration, and attenuation of OST1 and RbohD activity by S-nitrosylation [78,79,80].
ABA also stimulates increases in cytosolic Ca2+ concentration. This is achieved by the release of Ca2+ from intracellular stores and Ca2+ influx into the cell via inward Ca2+ transporters [31,113,114,115,116,117,118]. One of the known mechanisms is the activation of hyperpolarization-activated Ca2+-permeable ICa channels by ROS (which is in turn increased by ABA, as described above) [58,119,120]. The elevated Ca2+ concentration activates CBL1 or CBL9, both of which form a complex with CIPK26 and phosphorylate RbohF, forming a positive feedback loop in ROS production [48,121]. Another group of Ca2+-activated kinases, CPK4, 5, 6 and 11, regulates RbohD in a similar manner [122,123]. Accumulation of ROS promotes synthesis of NO, which is capable of releasing Ca2+ from intracellular Ca2+ stores [72,124,125]. These mechanisms allow the increase of cytosolic Ca2+ concentration and the activation of various Ca2+ dependent kinases in guard cell, which subsequently phosphorylate and activate SLAC1. To date, the following kinases are known to participate in activation of SLAC1: CPK3, 6, 21, 23, CIPK23 (with CBL1 or CBL9) and CIPK11 (with CBL5) [55,61,82,83,126]. All these kinases are confirmed to activate SLAC1 in Xenopus oocyte, and they recognize different phosphorylation sites from OST1 (for instance, CPK6 phosphorylates Ser59 of SLAC1) [61,83]. However these kinases, like OST1, are confirmed to be inhibited by ABI1 and ABI2, suggesting a complex crosstalk between SLAC1-activating kinases [61,82,83,127]. The four CPKs seem to display differences in Ca2+ affinity, since CPK6 and CPK23 enable SLAC1 to emit anion currents at resting Ca2+ concentration in oocytes, while CPK3 and CPK21 requires deletion of their EF-hands (which renders them constitutively active) [82,126]. Patch clamp analysis revealed the severe reduction of S-type anion channel current amplitude in guard cell of cpk23 [82]. In addition, the ABA-induced activation of guard cell S-type anion channels was disrupted in cpk3cpk6 double or cpk5cpk6cpk11cpk23 quadruple mutant [128,129]. While cpk3cpk6 and cpk5cpk6cpk11cpk23 mutant plants showed impaired ABA-induced stomatal closure, stomatal behavior in cipk23 and cbl1cbl9 was somehow the opposite, displaying reduced leaf water transpiration and enhanced drought tolerance [55,109,128,129]. The effect of cpk23 mutation is still under debate since conflicting results are reported [130,131]. cpk21 mutant showed enhanced tolerance in osmotic stress and no change in stomatal conductance, even though CPK21 was confirmed to activate GORK, an outward guard cell K+ channel that works synergistically with SLAC1 upon stomatal closure [111,131,132]. These somewhat confusing results can be explained by functional overlapping and compensation of CPKs. For example, gene expression of CPK23 is upregulated in cpk21 mutant plant [132]. As for CIPK23, this kinase is known to activate various channels other than SLAC1, including inward K+ channel AKT1 [108,109,110]. It was therefore speculated, that CIPK23 activates other channels like AKT1 rather than SLAC1 in vivo, resulting in negative regulation of ABA signaling in guard cells [3,45]. Recently, N-myristoylation and S-acylation at the N-terminus of CPK or CBL, was identified as an essential modification of CPK6 and CBL5-CIPK11 upon their activation of SLAC1, in terms of their PM recruitment [55]. Conservation of N-myristoylation and S-acylation motif among CPKs and CBLs suggest this mechanism as a common requirement for ion channel regulation by these kinases at the membrane [133,134,135,136,137].
In addition to ABA, methyl jasmonate (MeJA) and salicylic acid (SA) also induces stomatal closure via the regulation of S-type anion channel (Figure 1). MeJA-induced stomatal closure is dependent on the activation of guard cell PM H+-ATPase, a process that is mediated by F-box protein COI1 (Coronatine-insensitive 1) [138]. This promotes hyperpolarization of PM and activation of PM Ca2+ channels, resulting in an increase of cytosolic Ca2+. The elevated Ca2+ level results in activation of CPK6, though not of CPK3, 4 or 11, and the consequent SLAC1 activation [139]. MPK9, MPK12 and GHR1 also participate in SLAC1 activation in this pathway, possibly as a consequence of RbohD/RbohF-mediated ROS production by elevated Ca2+ [49,64]. Similarly, SA-induced stomatal closure involves ROS production, followed by SLAC1 activation via CPK3, 6, MPK9, MPK12, and GHR1 [54,64,140]. However, this pathway is unique in the way that ROS is produced: it features extracellular ROS produced by SHAM (salicylhydroxamic acid)-sensitive peroxidase, which is then diffused into guard cells [141].
Because stomata work as a major gateway for CO2 influx, elevated CO2 levels also promote stomatal closure (Figure 1) [39,45]. In this regulation pathway, CO2 imported from stomatal pore is first converted into HCO3− by two kinds of carbonic anhydrases (CAs), βCA1 and βCA4. Exposure to high CO2 condition elevates the intracellular HCO3− concentration, stimulating an HCO3− sensing component named RHC1 (Resistant to high carbon dioxide 1). This induces the formation of a complex between CA and RHC1, which enables the interaction with, and the subsequent inactivation of HT1 kinase (High leaf temperature 1e), an inhibitor of OST1 and GHR1 [45,81,142,143,144,145,146]. This mechanism would allow SLAC1 activation and the consequent stomatal closure under high CO2 condition. In a recent study, CO2-permeable aquaporin PIP2;1 was identified as an upstream regulator of βCA1 and βCA4, and MPK4 and MPK12 as additional intermediates for HT1 regulation by HCO3− [81,147].
Immediate stomatal closure is necessary during bacterial invasion, for stomata might serve as an entrance for bacteria [148,149]. Melotto et al. revealed that plant closes stomata when exposed to P. syringae, E. coli and PAMPs (pathogen-associated molecular pattern) [150]. This mechanism was predicted to involve a signal transduction from a receptor like kinase FLS2 (which recognizes flg22, a 22-amino-acid residue stretch of the flagellin protein from P. syringae) to some kinases that activate SLAC1 [148,151,152,153]. In recent years it was indicated that OST1, not CPKs, was responsible for this pathogen-induced stomatal closure mediated by SLAC1 [154]. However, it was also confirmed that NADPH oxidases and ABI1 were unnecessary for this pathway, leaving a missing link between FLS2 and OST1 [154]. Several studies report the formation of a complex between FLS2 and BAK1, a possible activator of OST1, so it can be hypothesized that BAK1 mediates the bacterial resistance signal from FLS2 to OST1 [68,84,85]. On the other hand, several researches proposed alternative pathways. Recent research reported that another FLS2-associated kinase, BIK1, directly phosphorylates RbohD, suggesting an OST1-independent ROS production pathway [86,87]. This seem to hold the truth for stomatal closure by another pathogen responsive component, danger-associated peptides (DAMPs), which was confirmed not to require OST1 [155]. Montillet et al. showed another OST1-independent pathway which involves the oxylipin production induced by lipoxygenase 1 (LOX1), resulting in an increased amount of SA (which might be consistent with the reports that MPK9 and MPK12 function in biotic stress response) [54,156,157]. Su et al. proposed a pathway insensitive to coronatine, a compound produced by pathogens, which promotes ‘reopening’ of stomata [153,158]. This model considers the activation of MPK3 and MPK6 and the induced malate/citrate metabolism as key features. However, direct connection to stomatal closure is still under discussion [158].
3. SLAHs (SLAC1 Homologues) and Nitrate Transporters
In the Arabidopsis genome, four SLAC1 homologue genes (SLAH1-4) are present [38,39]. When focusing on stomatal movements, SLAH3 protein is particularly important among these four, since it is the only SLAH3 confirmed to be expressed in guard cell [88,107]. Unlike SLAC1, which is permeable to both chloride and nitrate, permeability of SLAH3 is strongly restricted to nitrate [88]. Guard cell protoplasts from slac1-3 mutant plants elicited S-type anion channel current in nitrate-based buffer, but not from the slah3-1 mutant, suggesting SLAH3, but not SLAC1, is the key component of nitrate-mediated stomatal regulation [88]. This indicates the requirement of both SLAC1 and SLAH3 for full stomatal function, as implied in some studies [154,155]. Most of the Ca2+ related SLAC1-activating kinases, including CPK3, CPK6, CPK21, CPK23, and CBL1/CBL9-CIPK23, can also activate SLAH3 [83,88,89]. However, (and significantly), SLAH3 was insensitive to OST1, a major activator of SLAC1 [88]. Surprisingly, it was reported that SLAH3, as well as SLAC1, physically interacts with and inhibits an inward K+ channel KAT1 [107]. Taken together, stomatal closure pathway by SLAH3, even though similar to that of SLAC1, harbors some unique features (Figure 1).
Cubero-Font et al. discovered that SLAH1 gene encodes a channel subunit which forms a heteromer with SLAH3, and renders it permeable to nitrate and chloride [159]. Although no evidence of SLAH1 expression in guard cell have arisen so far, it is interesting to note that SLAH1 expression driven by SLAC1 promoter can complement the stomatal phenotypes of slac1 mutation [39,159]. SLAH2, on the other hand, is expressed mainly in roots, and shows S-type channel activity with strict nitrate selectivity [44]. CBL1-CIPK23 and several CPKs, including CPK3 and 21, were identified as the activator of this nitrate channel [44,160]. To this date, data on the function and expression of SLAH4 awaits discovery.
In 2003, Guo et al. demonstrated that CHL1 (also referred to as AtNRT1.1 or AtNPF6.3), a dual-affinity nitrate transporter, is expressed in guard cell and functions in stomatal opening and guard cell nitrate accumulation [161]. CBL1/CBL9-CIPK23 phosphorylates CHL1 and converts its nitrate transport from low-affinity mode to high-affinity mode (which, in turn, results in reduced nitrate intake under sufficient nitrate concentration) [90,91,92,93]. The behavior of CHL1 in stomata has not been reported extensively. The fact that they are both regulated by CBL1/9-CIPK23 suggests that there might be a crosstalk between CHL1 and SLAH (Figure 1).
4. Malate Transporters
Even after SLAC1 was identified as a guard cell S-type anion channel, the origin of R-type anion channel currents had remained unknown [38,39]. Then in 2010, several researches identified AtALMT12, a member of aluminum-activated malate transporter family, as a major component of the guard cell R-type anion channels [46,162,163]. AtALMT12 represents an anion channel permeable to malate, chloride and nitrate, and unlike SLAC1 it does not require any kinases for activation [162,163]. However, coexpression of OST1 in Xenopus oocytes resulted in further enhancement of AtALMT12 current [97]. AtALMT12 is different from its homologue AtALMT1 in that its activity was not stimulated by Al3+ [163,164]. atalmt12 mutant plants showed partially impaired stomatal closure in response to various stimuli such as CO2, ABA and Ca2+ [162,163]. AtALMT12 shows rapid inactivation at hyperpolarized membrane potential, in which its cytosolic C-terminal domain serves as a voltage sensor [165].
Three other members of ALMT family reside in Arabidopsis guard cell tonoplast; AtALMT4, 6 and 9 (Figure 1) [95,96,166,167,168]. AtALMT6 was identified as a Ca2+-activated channel permeable to malate and fumarate, and its activity modulated by vacuolar pH [95]. This channel can mediate malate influx upon tonoplast hyperpolarization, and efflux upon depolarization [95]. Atalmt6 mutant plant displayed reduced malate current in guard cell vacuole. However, no obvious phenotypic difference was observed compared to WT plants [95]. AtALMT9, on the other hand, acts as a chloride efflux channel activated by cytosolic malate, and the phenotype of the mutant plant evidenced its role in stomatal opening [96]. Recently, AtALMT4 was shown to be another tonoplast ALMT, that can mediate anion influx and efflux, regulated by phosphorylation at Ser382 residue [94]. Though its function shows some similarity to AtALMT6, Atalmt4 mutant plant shows obvious impairment in ABA-induced stomatal closure [94].
The significance of malate during stomatal movements has been implied decades before the identification of ALMTs described above [47,169,170,171,172]. This includes malate release from mesophyll cells and malate synthesis in guard cells [22,47,171,172]. In 2008, an ABC transporter AtABCB14, was reported to mediate malate influx across guard cell PM, and confirmed to participate in stomatal opening [98]. Together, these data suggest an elaborate regulation of stomatal movement by malate.
5. Other Anion Channels Involved in Stomatal Closure
In addition to the channels described above, several anion channels from other families have been shown to compete in stress-induced stomatal movement. CLC is a family of chloride channels that can be found ubiquitously among bacteria, animals, and plants [46,173,174,175,176]. Seven members of CLC gene, AtCLCa-g, are present in Arabidopsis genome [177,178]. Among these seven, AtCLCc is particularly expressed in guard cell tonoplast. Though its detailed function remains unveiled, the phenotype of clcc mutants postulate its participation in stomatal response to ABA and NaCl through modulation of chloride/nitrate homeostasis [102]. Wege et al. stated that another CLC member AtCLCa, originally identified as a tonoplast nitrate/proton antiporter, was required for ABA-induced stomatal closure or inhibition of stomatal opening [99,100]. AtCLCa mediates nitrate influx at hyperpolarization and efflux upon depolarization [99,100]. Although ATP binding to the C-terminus of AtCLCa inhibits its transport activity, its activity can be resumed by phosphorylation via OST1, suggesting some crosstalk between SLAC1 and AtALMT12 regulation [100,101,168]. Two members from Arabidopsis detoxification efflux carrier (DTX)/Multidrug and toxic compound extrusion (MATE) family, DTX33 and 35, were recently identified as an additional anion channel residing in guard cell tonoplast [103,104,105]. Both channels exhibit vacuolar chloride influx in various types of cells, including guard cell, and mutation of their genes results in impaired stomatal opening [105]. Andrés et al. reported that the dynamic structure change of guard cell vacuole itself is necessary for proper regulation of stomata movement, and that this is mediated by tonoplast K+/H+ antiporter NHX1 and NHX2, and, possibly, by the yet unidentified Na+/K+ antiporter [179]. It was later proposed that CBL2, CBL3, CIPK9 and CIPK17 might contribute in this NHX1/2-mediated convolution of vacuole [112].
Studies from the 1980s demonstrated that SO2 can also promote stomatal closure, which was most recently concluded as being a result of non-apoptotic cell death caused by the accumulation of H2SO3 [180,181,182]. Another research implicates sulfate as an important element for drought-induced stomatal closure [106]. Sulfate, uptaken from roots through sulfate transporter SULTRs, had long been known as an essential macronutrient for plant growth and development [27,28,183]. SULTR family is divided into five groups [184]. Recently the essential role of sulfate in ABA synthesis was elucidated, suggesting the participation of SULTRs in abiotic stress response [29,30,185,186]. Malcheska et al. reported stomatal closure induced by apoplastic sulfate, and activation of AtALMT12 by sulfate application [106]. This study also proposed that sulfate induces enhanced ABA synthesis inside the guard cell, possibly involving chloroplastic SULTR3;1 as a key transporter (Figure 1) [106].
6. Conclusions
Stomatal movement triggered by biotic and abiotic stress is regulated by an intricate mechanism involving various guard cell expressed ion channels and their regulator components (kinases, phytohormone receptor, etc.). The circulation and accumulation of anions in plants require a large number of transport systems. The extensive identification on anion channels and transporters described in this mini-review has filled in many of the gaps which had precluded the full understanding of the mechanism of ion homeostasis and cellular adaptation against harsh salinity stress. Further progress is a necessary demand for the successful modification of plant stress tolerance to global environmental changes.
Acknowledgments
We thank Marinela Anderca for critical reading of the manuscript.
Author Contributions
Conceptualization, S.S.; Writing—original draft preparation, S.S.; Writing—review and editing, N.U.; Funding acquisition, S.S. and N.U.
Funding
This work was supported by Grants-in-Aid for Scientific Research (16H06558 and 16H04906) to N.U., and (18H05996) to S.S. from the MEXT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Szyroki A., Ivashikina N., Dietrich P., Roelfsema M.R.G., Ache P., Reintanz B., Deeken R., Godde M., Felle H., Steinmeyer R., et al. KAT1 is not essential for stomatal opening. Proc. Natl. Acad. Sci. USA. 2001;98:2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma T., Dreyer I., Riedelsberger J. The role of K(+) channels in uptake and redistribution of potassium in the model plant Arabidopsis thaliana. Front. Plant Sci. 2013;4:224. doi: 10.3389/fpls.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieves-Cordones M., Caballero F., Martínez V., Rubio F. Disruption of the arabidopsis thaliana inward-rectifier K + channel AKT1 improves plant responses to water stress. Plant Cell Physiol. 2012;53:423–432. doi: 10.1093/pcp/pcr194. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura R.L., McKendree W.L., Hirsch R.E., Sedbrook J.C., Gaber R.F., Sussman M.R. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilot G., Lacombe B., Gaymard F., Chérel I., Boucherez J., Thibaud J.B., Sentenac H. Guard Cell Inward K+ Channel Activity in Arabidopsis Involves Expression of the Twin Channel Subunits KAT1 and KAT2. J. Biol. Chem. 2001;276:3215–3221. doi: 10.1074/jbc.M007303200. [DOI] [PubMed] [Google Scholar]
- 6.Lebaudy A., Vavasseur A., Hosy E., Dreyer I., Leonhardt N., Thibaud J.-B., Véry A.-A., Simonneau T., Sentenac H. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. USA. 2008;105:5271–5276. doi: 10.1073/pnas.0709732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimazaki K., Doi M., Assmann S.M., Kinoshita T. Light Regulation of Stomatal Movement. Annu. Rev. Plant Biol. 2007;58:219–247. doi: 10.1146/annurev.arplant.57.032905.105434. [DOI] [PubMed] [Google Scholar]
- 8.Kwak J.M., Murata Y., Baizabal-Aguirre V.M., Merrill J., Wang M., Kemper A., Hawke S.D., Tallman G., Schroeder J.I. Dominant Negative Guard Cell K+ Channel Mutants Reduce Inward-Rectifying K+ Currents and Light-Induced Stomatal Opening in Arabidopsis. Plant Physiol. 2001;127:473–485. doi: 10.1104/pp.010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B., Tester M., Gilliham M. Chloride on the Move. Trends Plant Sci. 2017;22:236–248. doi: 10.1016/j.tplants.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Teakle N.L., Tyerman S.D. Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ. 2010;33:566–589. doi: 10.1111/j.1365-3040.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 11.White P.J., Broadley M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001;88:967–988. doi: 10.1006/anbo.2001.1540. [DOI] [Google Scholar]
- 12.Krapp A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015;25:115–122. doi: 10.1016/j.pbi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Xuan W., Beeckman T., Xu G. Plant nitrogen nutrition: sensing and signaling. Curr. Opin. Plant Biol. 2017;39:57–65. doi: 10.1016/j.pbi.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Schulze J., Tesfaye M., Litjens R.H.M.G., Bucciarelli B., Trepp G., Miller S., Samac D., Allan D., Vance C.P. Malate plays a central role in plant nutrition. Plant Soil. 2002;247:133–139. doi: 10.1023/A:1021171417525. [DOI] [Google Scholar]
- 15.Finkemeier I., Sweetlove L. The role of malate in plant homeostasis. F1000 Biol. Rep. 2009;3:10–12. doi: 10.3410/B1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plaxton W.C. The Organization and Regulation of Plant Glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- 17.Zell M.B., Fahnenstich H., Maier A., Saigo M., Voznesenskaya E.V., Edwards G.E., Andreo C., Schleifenbaum F., Zell C., Drincovich M.F., et al. Analysis of Arabidopsis with Highly Reduced Levels of Malate and Fumarate Sheds Light on the Role of These Organic Acids as Storage Carbon Molecules. Plant Physiol. 2010;152:1251–1262. doi: 10.1104/pp.109.151795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernie A.R., Martinoia E. Malate. Jack of all trades or master of a few? Phytochemistry. 2009;70:828–832. doi: 10.1016/j.phytochem.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Nunes-Nesi A., Araújo W.L., Obata T., Fernie A.R. Regulation of the mitochondrial tricarboxylic acid cycle. Curr. Opin. Plant Biol. 2013;16:335–343. doi: 10.1016/j.pbi.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Leegood R.C. C4 photosynthesis: principles of CO2 concentration and prospects for its introduction into C3 plants. J. Exp. Bot. 2002;53:581–590. doi: 10.1093/jexbot/53.369.581. [DOI] [PubMed] [Google Scholar]
- 21.Meister M., Agostino A., Hatch M.D. The roles of malate and aspartate in C4photosynthetic metabolism of Flaveria bidentis (L.) Planta. 1996;199:262–269. doi: 10.1007/BF00196567. [DOI] [Google Scholar]
- 22.Jeanneau M. Manipulating PEPC levels in plants. J. Exp. Bot. 2002;53:1837–1845. doi: 10.1093/jxb/erf061. [DOI] [PubMed] [Google Scholar]
- 23.Lüttge U., Nobel P.S. Day-night variations in malate concentration, osmotic pressure, and hydrostatic pressure in Cereus validus. Plant Physiol. 1984;75:804–807. doi: 10.1104/pp.75.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith R.G., Gauthier D.A., Dennis D.T., Turpin D.H. Malate- and pyruvate-dependent Fatty Acid synthesis in leucoplasts from developing castor endosperm. Plant Physiol. 1992;98:1233–1238. doi: 10.1104/pp.98.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneidereit J., Häusler R.E., Fiene G., Kaiser W.M., Weber A.P.M. Antisense repression reveals a crucial role of the plastidic 2-oxoglutarate/malate translocator DiT1 at the interface between carbon and nitrogen metabolism. Plant J. 2006;45:206–224. doi: 10.1111/j.1365-313X.2005.02594.x. [DOI] [PubMed] [Google Scholar]
- 26.Palmer A.J., Baker A., Muench S.P. The varied functions of aluminium-activated malate transporters-much more than aluminium resistance. Biochem. Soc. Trans. 2016;44:856–862. doi: 10.1042/BST20160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopriva S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann. Bot. 2006;97:479–495. doi: 10.1093/aob/mcl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gigolashvili T., Kopriva S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014;5:1–16. doi: 10.3389/fpls.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendel R.R. The molybdenum cofactor. J. Biol. Chem. 2013;288:13165–13172. doi: 10.1074/jbc.R113.455311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao M.J., Wang Z., Zhao Q., Mao J.L., Speiser A., Wirtz M., Hell R., Zhu J.K., Xiang C. Bin Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J. 2014;77:604–615. doi: 10.1111/tpj.12407. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder J.I., Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989 doi: 10.1038/338427a0. [DOI] [Google Scholar]
- 32.Hedrich R., Busch H., Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder J.I., Keller B.U. Two types of anion channel currents in guard cells with distinct voltage regulation. Proc. Natl. Acad. Sci. USA. 1992;89:5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linder B., Raschke K. A slow anion channel in guard cells, activating at large hyperpolarization, may be principal for stomatal closing. FEBS Lett. 1992;313:27–30. doi: 10.1016/0014-5793(92)81176-M. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder J., Schmidt C., Sheaffer J. Identification of High-Affinity Slow Anion Channel Blockers and Evidence for Stomatal Regulation by Slow Anion Channels in Guard Cells. Plant Cell. 1993;5:1831–1841. doi: 10.1105/tpc.5.12.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pei Z.-M., Kuchitsu K., Ward J.M., Schwarz M., Schroeder J.I. Differential Abscisic Acid Regulation of Guard Cell Slow Anion Channels in Arabidopsis Wild-Type and abi1 and abi2 Mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kangasjärvi J., Jaspers P., Kollist H. Signalling and cell death in ozone-exposed plants. Plant Cell Environ. 2005;28:1021–1036. doi: 10.1111/j.1365-3040.2005.01325.x. [DOI] [Google Scholar]
- 38.Vahisalu T., Kollist H., Wang Y.-F., Nishimura N., Chan W.-Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., Uchimiya H., Hashimoto M., Iba K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y.-H., Hu L., Punta M., Bruni R., Hillerich B., Kloss B., Rost B., Love J., Siegelbaum S.A., Hendrickson W.A. Homologue structure of the SLAC1 anion channel for closing stomata in leaves. Nature. 2010;467:1074–1080. doi: 10.1038/nature09487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du Q.S., Fan X.W., Wang C.H., Huang R.B. A possible CO2 conducting and concentrating mechanism in plant stomata SLAC1 channel. PLoS ONE. 2011;6:e24264. doi: 10.1371/journal.pone.0024264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt C., Schelle I., Liao Y.J., Schroeder J.I. Strong Regulation of Slow Anion Channels and Abscisic-Acid Signaling in Guard-Cells by Phosphorylation and Dephosphorylation Events. Proc. Natl. Acad. Sci. USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J., Wang X.-Q., Watson M.B., Assmann S.M. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- 44.Maierhofer T., Lind C., Hüttl S., Scherzer S., Papenfuß M., Simon J., Al-Rasheid K.A.S., Ache P., Rennenberg H., Hedrich R., et al. A Single-Pore Residue Renders the Arabidopsis Root Anion Channel SLAH2 Highly Nitrate Selective. Plant Cell. 2014;3:1–15. doi: 10.1105/tpc.114.125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim T.-H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010;61:561–591. doi: 10.1146/annurev-arplant-042809-112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbier-Brygoo H., De Angeli A., Filleur S., Frachisse J.-M., Gambale F., Thomine S., Wege S. Anion Channels/Transporters in Plants: From Molecular Bases to Regulatory Networks. Annu. Rev. Plant Biol. 2011;62:25–51. doi: 10.1146/annurev-arplant-042110-103741. [DOI] [PubMed] [Google Scholar]
- 47.Daszkowska-Golec A., Szarejko I. Open or Close the Gate – Stomata Action Under the Control of Phytohormones in Drought Stress Conditions. Front. Plant Sci. 2013;4:1–16. doi: 10.3389/fpls.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittler R., Blumwald E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell. 2015;27:64–70. doi: 10.1105/tpc.114.133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khokon M.A.R., Salam M.A., Jammes F., Ye W., Hossain M.A., Uraji M., Nakamura Y., Mori I.C., Kwak J.M., Murata Y. Two guard cell mitogen-activated protein kinases, MPK9 and MPK12, function in methyl jasmonate-induced stomatal closure in Arabidopsis thaliana. Plant Biol. 2015;17:946–952. doi: 10.1111/plb.12321. [DOI] [PubMed] [Google Scholar]
- 50.Munemasa S., Hauser F., Park J., Waadt R., Brandt B., Schroeder J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015;28:154–162. doi: 10.1016/j.pbi.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sierla M., Waszczak C., Vahisalu T., Kangasjärvi J. Reactive Oxygen Species in the Regulation of Stomatal Movements. Plant Physiol. 2016;171:1569–1580. doi: 10.1104/pp.16.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hedrich R., Geiger D. Biology of SLAC1-type anion channels – from nutrient uptake to stomatal closure. New Phytol. 2017 doi: 10.1111/nph.14685. [DOI] [PubMed] [Google Scholar]
- 53.Melotto M., Zhang L., Oblessuc P.R., He S.Y. Stomatal Defense a Decade Later. Plant Physiol. 2017;174:561–571. doi: 10.1104/pp.16.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prodhan M.Y., Munemasa S., Nahar M.N.-E.-N., Nakamura Y., Murata Y. Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol. 2018;178:441–450. doi: 10.1104/pp.18.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saito S., Hamamoto S., Moriya K., Matsuura A., Sato Y., Muto J., Noguchi H., Dong Q., Held K., Kudla J., et al. N-myristoylation and S-acylation are common modifications of Ca2+-regulated Arabidopsis kinases and are required for activation of the SLAC1 anion channel. New Phytol. 2018;218:1504–1521. doi: 10.1111/nph.15053. [DOI] [PubMed] [Google Scholar]
- 56.Mustilli A.-C., Merlot S., Vavasseur A., Fenzi F., Giraudat J. Arabidopsis OST1 Protein Kinase Mediates the Regulation of Stomatal Aperture by Abscisic Acid and Acts Upstream of Reactive Oxygen Species Production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A.S., et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murata Y., Pei Z., Mori I.C., Schroeder J. Abscisic Acid Activation of Plasma Membrane Ca2+ Channels in Guard Cells Requires Cytosolic NAD (P) H and Is Differentially Disrupted Upstream and Downstream of Reactive Oxygen Species Production in abi1-1 and abi2-1 Protein Phosphatase 2C Mutants. Plant Cell. 2001;13:2513–2523. doi: 10.2307/3871591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida R., Umezawa T., Mizoguchi T., Takahashi S., Takahashi F., Shinozaki K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J. Biol. Chem. 2006;281:5310–5318. doi: 10.1074/jbc.M509820200. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura N., Sarkeshik A., Nito K., Park S.Y., Wang A., Carvalho P.C., Lee S., Caddell D.F., Cutler S.R., Chory J., et al. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brandt B., Brodsky D.E., Xue S., Negi J., Iba K., Kangasjarvi J., Ghassemian M., Stephan A.B., Hu H., Schroeder J.I. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA. 2012;109:10593–10598. doi: 10.1073/pnas.1116590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., Hibi T., Taniguchi M., Miyake H., Goto D.B., et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 2009;424:439–448. doi: 10.1042/BJ20091221. [DOI] [PubMed] [Google Scholar]
- 63.Acharya B.R., Jeon B.W., Zhang W., Assmann S.M. Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 2013;200:1049–1063. doi: 10.1111/nph.12469. [DOI] [PubMed] [Google Scholar]
- 64.Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., Gong Z. A Plasma Membrane Receptor Kinase, GHR1, Mediates Abscisic Acid- and Hydrogen Peroxide-Regulated Stomatal Movement in Arabidopsis. Plant Cell. 2012;24:2546–2561. doi: 10.1105/tpc.112.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sirichandra C., Gu D., Hu H.C., Davanture M., Lee S., Djaoui M., Valot B., Zivy M., Leung J., Merlot S., et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009;583:2982–2986. doi: 10.1016/j.febslet.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 66.Vahisalu T., Puzõrjova I., Brosché M., Valk E., Lepiku M., Moldau H., Pechter P., Wang Y.S., Lindgren O., Salojärvi J., et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J. 2010;62:442–453. doi: 10.1111/j.1365-313X.2010.04159.x. [DOI] [PubMed] [Google Scholar]
- 67.Jammes F., Song C., Shin D., Munemasa S., Takeda K., Gu D., Cho D., Lee S., Giordo R., Sritubtim S., et al. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA. 2009;106:20520–20525. doi: 10.1073/pnas.0907205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shang Y., Dai C., Lee M.M., Kwak J.M., Nam K.H. BRI1-Associated Receptor Kinase 1 Regulates Guard Cell ABA Signaling Mediated by Open Stomata 1 in Arabidopsis. Mol. Plant. 2016;9:447–460. doi: 10.1016/j.molp.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 69.Bright J., Desikan R., Hancock J.T., Weir I.S., Neill S.J. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 70.Wilson I.D., Ribeiro D.M., Bright J., Confraria A., Harrison J., Barros R.S., Desikan R., Neill S.J., Hancock J.T. Role of nitric oxide in regulating stomatal apertures. Plant Signal. Behav. 2009;4:467–469. doi: 10.4161/psb.4.5.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neill S., Barros R., Bright J., Desikan R., Hancock J., Harrison J., Morris P., Ribeiro D., Wilson I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 72.Gayatri G., Agurla S., Raghavendra A.S. Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 2013 doi: 10.3389/fpls.2013.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laxalt A.M., García-Mata C., Lamattina L. The Dual Role of Nitric Oxide in Guard Cells: Promoting and Attenuating the ABA and Phospholipid-Derived Signals Leading to the Stomatal Closure. Front. Plant Sci. 2016;7:2007–2010. doi: 10.3389/fpls.2016.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W., Qin C., Zhao J., Wang X. Phospholipase D 1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA. 2004;101:9508–9513. doi: 10.1073/pnas.0402112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y., Zhu H., Zhang Q., Li M., Yan M., Wang R., Wang L., Welti R., Zhang W., Wang X. Phospholipase D 1 and Phosphatidic Acid Regulate NADPH Oxidase Activity and Production of Reactive Oxygen Species in ABA-Mediated Stomatal Closure in Arabidopsis. Plant Cell Online. 2009;21:2357–2377. doi: 10.1105/tpc.108.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Distéfano A.M., García-Mata C., Lamattina L., Laxalt A.M. Nitric oxide-induced phosphatidic acid accumulation: A role for phospholipases C and D in stomatal closure. Plant Cell Environ. 2008;31:187–194. doi: 10.1111/j.1365-3040.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 77.Distéfano A.M., Scuffi D., García-Mata C., Lamattina L., Laxalt A.M. Phospholipase Dδ is involved in nitric oxide-induced stomatal closure. Planta. 2012;236:1899–1907. doi: 10.1007/s00425-012-1745-4. [DOI] [PubMed] [Google Scholar]
- 78.Yun B.W., Feechan A., Yin M., Saidi N.B.B., Le Bihan T., Yu M., Moore J.W., Kang J.G., Kwon E., Spoel S.H., et al. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature. 2011;478:264–268. doi: 10.1038/nature10427. [DOI] [PubMed] [Google Scholar]
- 79.Wang P., Du Y., Hou Y.-J., Zhao Y., Hsu C.-C., Yuan F., Zhu X., Tao W.A., Song C.-P., Zhu J.-K. Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc. Natl. Acad. Sci. USA. 2015;112:613–618. doi: 10.1073/pnas.1423481112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castillo M., Lozano-juste J., González-guzmán M., Rodriguez L., Rodriguez P.L., Leon H. Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling by nitric oxide in plants Inactivation of PYR/PYL/RCAR ABA receptors by tyrosine nitration may enable rapid inhibition of ABA signaling b. Sci. Signal. 2015;8:1–9. doi: 10.1126/scisignal.aaa7981. [DOI] [PubMed] [Google Scholar]
- 81.Hõrak H., Sierla M., Tõldsepp K., Wang C., Wang Y.-S., Nuhkat M., Valk E., Pechter P., Merilo E., Salojärvi J., et al. A Dominant Mutation in the HT1 Kinase Uncovers Roles of MAP Kinases and GHR1 in CO2-Induced Stomatal Closure. Plant Cell. 2016;28:2493–2509. doi: 10.1105/tpc.16.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A.S., Grill E., et al. Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA. 2010;107:8023–8028. doi: 10.1073/pnas.0912030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maierhofer T., Diekmann M., Offenborn J.N., Lind C., Bauer H., Hashimoto K., Al-Rasheid K.A.S., Luan S., Kudla J., Geiger D., et al. Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci. Signal. 2014;7:ra86. doi: 10.1126/scisignal.2005703. [DOI] [PubMed] [Google Scholar]
- 84.Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D.G., Felix G., Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 85.Sun Y., Li L., Macho A.P., Han Z., Hu Z., Zipfel C., Zhou J.M., Chai J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013;342:624–628. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- 86.Kadota Y., Sklenar J., Derbyshire P., Stransfeld L., Asai S., Ntoukakis V., Jones J.D., Shirasu K., Menke F., Jones A., et al. Direct Regulation of the NADPH Oxidase RBOHD by the PRR-Associated Kinase BIK1 during Plant Immunity. Mol. Cell. 2014;54:43–55. doi: 10.1016/j.molcel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 87.Li L., Li M., Yu L., Zhou Z., Liang X., Liu Z., Cai G., Gao L., Zhang X., Wang Y., et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe. 2014;15:329–338. doi: 10.1016/j.chom.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 88.Geiger D., Maierhofer T., Al-Rasheid K.A.S., Scherzer S., Mumm P., Liese A., Ache P., Wellmann C., Marten I., Grill E., et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci. Signal. 2011;4:ra32. doi: 10.1126/scisignal.2001346. [DOI] [PubMed] [Google Scholar]
- 89.Demir F., Horntrich C., Blachutzik J.O., Scherzer S., Reinders Y., Kierszniowska S., Schulze W.X., Harms G.S., Hedrich R., Geiger D., et al. Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc. Natl. Acad. Sci. USA. 2013;110:8296–8301. doi: 10.1073/pnas.1211667110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu K.H., Tsay Y.F. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003 doi: 10.1093/emboj/cdg118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. CHL1 Functions as a Nitrate Sensor in Plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 92.Parker J.L., Newstead S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature. 2014;507:68–72. doi: 10.1038/nature13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Léran S., Muños S., Brachet C., Tillard P., Gojon A., Lacombe B. Arabidopsis NRT1.1 is a bidirectional transporter involved in root-to-shoot Nitrate translocation. Mol. Plant. 2013;6:1984–1987. doi: 10.1093/mp/sst068. [DOI] [PubMed] [Google Scholar]
- 94.Eisenach C., Baetz U., Huck N.V., Zhang J., De Angeli A., Beckers G., Martinoia E. ABA-Induced Stomatal Closure Involves ALMT4, a Phosphorylation-Dependent Vacuolar Anion Channel of Arabidopsis. Plant Cell. 2017;29:tpc.00452.2017. doi: 10.1105/tpc.17.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meyer S., Scholz-Starke J., De Angeli A., Kovermann P., Burla B., Gambale F., Martinoia E. Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J. 2011;67:247–257. doi: 10.1111/j.1365-313X.2011.04587.x. [DOI] [PubMed] [Google Scholar]
- 96.De Angeli A., Zhang J., Meyer S., Martinoia E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 2013;4:1804–1810. doi: 10.1038/ncomms2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Imes D., Mumm P., Böhm J., Al-Rasheid K.A.S., Marten I., Geiger D., Hedrich R. Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J. 2013;74:372–382. doi: 10.1111/tpj.12133. [DOI] [PubMed] [Google Scholar]
- 98.Lee M., Choi Y., Burla B., Kim Y.Y., Jeon B., Maeshima M., Yoo J.Y., Martinoia E., Lee Y. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 2008;10:1217–1223. doi: 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- 99.De Angeli A., Monachello D., Ephritikhine G., Frachisse J.M., Thomine S., Gambale F., Barbier-Brygoo H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature. 2006;442:939–942. doi: 10.1038/nature05013. [DOI] [PubMed] [Google Scholar]
- 100.Wege S., De Angeli A., Droillard M.J., Kroniewicz L., Merlot S., Cornu D., Gambale F., Martinoia E., Barbier-Brygoo H., Thomine S., et al. Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci. Signal. 2014;7:1–11. doi: 10.1126/scisignal.2005140. [DOI] [PubMed] [Google Scholar]
- 101.De Angeli A., Moran O., Wege S., Filleur S., Ephritikhine G., Thomine S., Barbier-Brygoo H., Gambale F. ATP binding to the C terminus of the arabidopsis thaliana nitrate/proton antiporter, AtCLCa, regulates nitrate transport into plant vacuoles. J. Biol. Chem. 2009;284:26526–26532. doi: 10.1074/jbc.M109.005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jossier M., Kroniewicz L., Dalmas F., Le Thiec D., Ephritikhine G., Thomine S., Barbier-Brygoo H., Vavasseur A., Filleur S., Leonhardt N. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 2010;64:563–576. doi: 10.1111/j.1365-313X.2010.04352.x. [DOI] [PubMed] [Google Scholar]
- 103.Li L., He Z., Pandey G.K., Tsuchiya T., Luan S. Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J. Biol. Chem. 2002;277:5360–5368. doi: 10.1074/jbc.M108777200. [DOI] [PubMed] [Google Scholar]
- 104.Omote H., Hiasa M., Matsumoto T., Otsuka M., Moriyama Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 2006;27:587–593. doi: 10.1016/j.tips.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 105.Zhang H., Zhao F.-G., Tang R.-J., Yu Y., Song J., Wang Y., Li L., Luan S. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2017;114:E2036–E2045. doi: 10.1073/pnas.1616203114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malcheska F., Ahmad A., Batool S., Müller H.M., Ludwig-Müller J., Kreuzwieser J., Randewig D., Hänsch R., Mendel R.R., Hell R., et al. Drought-Enhanced Xylem Sap Sulfate Closes Stomata by Affecting ALMT12 and Guard Cell ABA Synthesis. Plant Physiol. 2017;174:798–814. doi: 10.1104/pp.16.01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang A., Ren H., Tan Y.-Q. S-type Anion Channels SLAC1 and SLAH3 Function as Essential Negative Regulators of Inward K+ Channels and Stomatal Opening in Arabidopsis. Plant Cell. 2016;4:305–314. doi: 10.1105/tpc.15.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 109.Cheong Y.H., Pandey G.K., Grant J.J., Batistic O., Li L., Kim B.G., Lee S.C., Kudla J., Luan S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007;52:223–239. doi: 10.1111/j.1365-313X.2007.03236.x. [DOI] [PubMed] [Google Scholar]
- 110.Lee S.C., Lan W.-Z., Kim B.-G., Li L., Cheong Y.H., Pandey G.K., Lu G., Buchanan B.B., Luan S. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. USA. 2007;104:15959–15964. doi: 10.1073/pnas.0707912104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Van Kleeff P.J.M., Gao J., Mol S., Zwart N., Zhang H., Li K.W., de Boer A.H. The Arabidopsis GORK K+-channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant Physiol. Biochem. 2018;125:219–231. doi: 10.1016/j.plaphy.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 112.Song S.-J., Feng Q.-N., Li C., Li E., Liu Q., Kang H., Zhang W., Zhang Y., Li S. A tonoplast-associated calcium-signaling module dampens ABA signaling during stomatal movement. Plant Physiol. 2018;177:1666–1678. doi: 10.1104/pp.18.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schroeder J.I., Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc. Natl. Acad. Sci. USA. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grabov A., Blatt M.R. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl. Acad. Sci. USA. 1998;95:4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leckie C.P., McAinsh M.R., Allen G.J., Sanders D., Hetherington A.M. Abscisic acid-induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Staxen I., Pical C., Montgomery L.T., Gray J.E., Hetherington A.M., McAinsh M.R. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA. 1999;96:1779–1784. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamilton D.W.A., Hills A., Kohler B., Blatt M.R. Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA. 2000;97:4967–4972. doi: 10.1073/pnas.080068897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MacRobbie E.A.C. ABA activates multiple Ca(2+) fluxes in stomatal guard cells, triggering vacuolar K(+)(Rb(+)) release. Proc. Natl. Acad. Sci. USA. 2000;97:12361–12368. doi: 10.1073/pnas.220417197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pei Z.M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 120.Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Angel Torres M., Dangl J.L., Bloom R.E., Bodde S., Jones J.D.G., Schroeder J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Drerup M.M., Schlücking K., Hashimoto K., Manishankar P., Steinhorst L., Kuchitsu K., Kudla J. The calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant. 2013;6:559–569. doi: 10.1093/mp/sst009. [DOI] [PubMed] [Google Scholar]
- 122.Boudsocq M., Willmann M.R., McCormack M., Lee H., Shan L., He P., Bush J., Cheng S.-H., Sheen J. Differential innate immune signalling via Ca2+ sensor protein kinases. Nature. 2010;464:418–422. doi: 10.1038/nature08794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kadota Y., Shirasu K., Zipfel C. Regulation of the NADPH Oxidase RBOHD during Plant Immunity. Plant Cell Physiol. 2015;56:1472–1480. doi: 10.1093/pcp/pcv063. [DOI] [PubMed] [Google Scholar]
- 124.Lamotte O., Courtois C., Dobrowolska G., Besson A., Pugin A., Wendehenne D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radic. Biol. Med. 2006;40:1369–1376. doi: 10.1016/j.freeradbiomed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 125.Jeandroz S., Lamotte O., Astier J., Rasul S., Trapet P., Besson-Bard A., Bourque S., Nicolas-Frances V., Ma W., Berkowitz G.A., et al. There’s More to the Picture Than Meets the Eye: Nitric Oxide Cross Talk with Ca2+ Signaling. Plant Physiol. 2013;163:459–470. doi: 10.1104/pp.113.220624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scherzer S., Maierhofer T., Al-Rasheid K.A.S., Geiger D., Hedrich R. Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol. Plant. 2012;5:1409–1412. doi: 10.1093/mp/sss084. [DOI] [PubMed] [Google Scholar]
- 127.Mao J., Manik S.M.N., Shi S., Chao J., Jin Y., Wang Q., Liu H. Mechanisms and physiological roles of the CBL-CIPK networking system in Arabidopsis thaliana. Genes. 2016;7:62. doi: 10.3390/genes7090062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mori I.C., Murata Y., Yang Y., Munemasa S., Wang Y.F., Andreoli S., Tiriac H., Alonso J.M., Harper J.F., Ecker J.R., et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+- permeable channels and stomatal closure. PLoS Biol. 2006;4:1749–1762. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brandt B., Munemasa S., Wang C., Nguyen D., Yong T., Yang P.G., Poretsky E., Belknap T.F., Waadt R., Alemán F., et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. Elife. 2015;4:1–25. doi: 10.7554/eLife.03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma S.Y., Wu W.H. AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol. Biol. 2007;65:511–518. doi: 10.1007/s11103-007-9187-2. [DOI] [PubMed] [Google Scholar]
- 131.Merilo E., Laanemets K., Hu H., Xue S., Jakobson L., Tulva I., Gonzalez-Guzman M., Rodriguez P.L., Schroeder J.I., Brosche M., et al. PYR/RCAR Receptors Contribute to Ozone-, Reduced Air Humidity-, Darkness-, and CO2-Induced Stomatal Regulation. Plant Physiol. 2013;162:1652–1668. doi: 10.1104/pp.113.220608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Franz S., Ehlert B., Liese A., Kurth J., Cazalé A.C., Romeis T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol. Plant. 2011;4:83–96. doi: 10.1093/mp/ssq064. [DOI] [PubMed] [Google Scholar]
- 133.Martín M.L., Busconi L. Membrane localization of a rice calcium-dependent protein kinase (CDPK) is mediated by myristoylation and palmitoylation. Plant J. 2000;24:429–435. doi: 10.1046/j.1365-313x.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 134.Boisson B., Giglione C., Meinnel T. Unexpected protein families including cell defense components feature in the N-myristoylome of a higher eukaryote. J. Biol. Chem. 2003;278:43418–43429. doi: 10.1074/jbc.M307321200. [DOI] [PubMed] [Google Scholar]
- 135.Podell S., Gribskov M. Predicting N-terminal myristoylation sites in plant proteins. BMC Genomics. 2004;5:37. doi: 10.1186/1471-2164-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Batistic O., Sorek N., Schültke S., Yalovsky S., Kudla J. Dual fatty acyl modification determines the localization and plasma membrane targeting of CBL/CIPK Ca2+ signaling complexes in Arabidopsis. Plant Cell. 2008;20:1346–1362. doi: 10.1105/tpc.108.058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mohanta T.K., Mohanta N., Mohanta Y.K., Parida P., Bae H. Genome-wide identification of Calcineurin B-Like (CBL) gene family of plants reveals novel conserved motifs and evolutionary aspects in calcium signaling events. BMC Plant Biol. 2015;15:189. doi: 10.1186/s12870-015-0543-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yan S., McLamore E.S., Dong S., Gao H., Taguchi M., Wang N., Zhang T., Su X., Shen Y. The role of plasma membrane H+-ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. Plant J. 2015;83:638–649. doi: 10.1111/tpj.12915. [DOI] [PubMed] [Google Scholar]
- 139.Munemasa S., Hossain M.A., Nakamura Y., Mori I.C., Murata Y. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 2011;155:553–561. doi: 10.1104/pp.110.162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khokon M.A.R., Salam M.A., Jammes F., Ye W., Hossain M.A., Okuma E., Nakamura Y., Mori I.C., Kwak J.M., Murata Y. MPK9 and MPK12 function in SA-induced stomatal closure in arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2017 doi: 10.1080/09168451.2017.1308244. [DOI] [PubMed] [Google Scholar]
- 141.Khokon M.A.R., Okuma E., Hossain M.A., Munemasa S., Uraji M., Nakamura Y., Mori I.C., Murata Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011;34:434–443. doi: 10.1111/j.1365-3040.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 142.Hashimoto M., Negi J., Young J., Israelsson M., Schroeder J.I., Iba K. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 2006;8:391–397. doi: 10.1038/ncb1387. [DOI] [PubMed] [Google Scholar]
- 143.Israelsson M., Siegel R.S., Young J., Hashimoto M., Iba K., Schroeder J.I. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr. Opin. Plant Biol. 2006;9:654–663. doi: 10.1016/j.pbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 144.Hu H., Boisson-Dernier A., Israelsson-Nordström M., Böhmer M., Xue S., Ries A., Godoski J., Kuhn J.M., Schroeder J.I. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat. Cell Biol. 2010;12:87–93. doi: 10.1038/ncb2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matrosova A., Bogireddi H., Mateo-Peñas A., Hashimoto-Sugimoto M., Iba K., Schroeder J.I., Israelsson-Nordström M. The HT1 protein kinase is essential for red light-induced stomatal opening and genetically interacts with OST1 in red light and CO2-induced stomatal movement responses. New Phytol. 2015;208:1126–1137. doi: 10.1111/nph.13566. [DOI] [PubMed] [Google Scholar]
- 146.Tian W., Hou C., Ren Z., Pan Y., Jia J., Zhang H., Bai F., Zhang P., Zhu H., He Y., et al. A molecular pathway for CO2 response in Arabidopsis guard cells. Nat. Commun. 2015;6:6057. doi: 10.1038/ncomms7057. [DOI] [PubMed] [Google Scholar]
- 147.Wang C., Hu H., Qin X., Zeise B., Xu D., Rappel W.-J., Boron W.F., Schroeder J.I. Reconstitution of CO2 Regulation of SLAC1 Anion Channel and Function of CO2-Permeable PIP2;1 Aquaporin as CARBONIC ANHYDRASE4 Interactor. Plant Cell. 2016;28:568–582. doi: 10.1105/tpc.15.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Underwood W., Melotto M., He S.Y. Role of plant stomata in bacterial invasion. Cell. Microbiol. 2007;9:1621–1629. doi: 10.1111/j.1462-5822.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- 149.Melotto M., Underwood W., He S.Y. Role of Stomata in Plant Innate Immunity and Foliar Bacterial Diseases. Annu. Rev. Phytopathol. 2009;46:101–122. doi: 10.1146/annurev.phyto.121107.104959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Melotto M., Underwood W., Koczan J., Nomura K., He S.Y. Plant Stomata Function in Innate Immunity against Bacterial Invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 151.Felix G., Duran J.D., Volko S., Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313X.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 152.Gómez-Gómez L., Boller T. FLS2: An LRR Receptor-like Kinase Involved in the Perception of the Bacterial Elicitor Flagellin in Arabidopsis. Mol. Cell. 2000;5:1003–1011. doi: 10.1016/S1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 153.Schulze-Lefert P., Robatzek S. Plant Pathogens Trick Guard Cells into Opening the Gates. Cell. 2006;126:831–834. doi: 10.1016/j.cell.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 154.Guzel Deger A., Scherzer S., Nuhkat M., Kedzierska J., Kollist H., Brosché M., Unyayar S., Boudsocq M., Hedrich R., Roelfsema M.R.G. Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol. 2015;208:162–173. doi: 10.1111/nph.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng X., Kang S., Jing Y., Ren Z., Li L., Zhou J.-M., Berkowitz G., Shi J., Fu A., Lan W., et al. Danger-Associated Peptides Close Stomata by OST1-Independent Activation of Anion Channels in Guard Cells. Plant Cell. 2018;30:1132–1146. doi: 10.1105/tpc.17.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Montillet J.L., Leonhardt N., Mondy S., Tranchimand S., Rumeau D., Boudsocq M., Garcia A.V., Douki T., Bigeard J., Laurière C., et al. An Abscisic Acid-Independent Oxylipin Pathway Controls Stomatal Closure and Immune Defense in Arabidopsis. PLoS Biol. 2013;11:13–15. doi: 10.1371/journal.pbio.1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jammes F., Yang X., Xiao S., Kwak J.M. Two arabidopsis guard cell-preferential MAPK genes, MPK9 and MPK12, function in biotic stress response. Plant Signal. Behav. 2011;6:1875–1878. doi: 10.4161/psb.6.11.17933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Su J., Zhang M., Zhang L., Sun T., Liu Y., Lukowitz W., Xu J., Zhang S. Regulation of Stomatal Immunity by Interdependent Functions of a Pathogen-Responsive MPK3/MPK6 Cascade and Abscisic Acid. Plant Cell. 2017;29:526–542. doi: 10.1105/tpc.16.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cubero-Font P., Maierhofer T., Jaslan J., Rosales M.A., Espartero J., Díaz-Rueda P., Müller H.M., Hürter A.-L., AL-Rasheid K.A.S., Marten I., et al. Silent S-Type Anion Channel Subunit SLAH1 Gates SLAH3 Open for Chloride Root-to-Shoot Translocation. Curr. Biol. 2016:1–8. doi: 10.1016/j.cub.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 160.Yao F.Y., Qi G.N., Hussain J. Investigation of the regulation mechanism of Arabidopsis thaliana anion channel SLAH2. Turk. J. Botany. 2017 doi: 10.3906/bot-1702-23. [DOI] [Google Scholar]
- 161.Guo F.-Q., Young J., Crawford N.M. The Nitrate Transporter AtNRT1.1 (CHL1) Functions in Stomatal Opening and Contributes to Drought Susceptibility in Arabidopsis. Plant Cell. 2003;15:107–117. doi: 10.1105/tpc.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sasaki T., Mori I.C., Furuichi T., Munemasa S., Toyooka K., Matsuoka K., Murata Y., Yamamoto Y. Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol. 2010;51:354–365. doi: 10.1093/pcp/pcq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meyer S., Mumm P., Imes D., Endler A., Weder B., Al-Rasheid K.A.S., Geiger D., Marten I., Martinoia E., Hedrich R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010;63:1054–1062. doi: 10.1111/j.1365-313X.2010.04302.x. [DOI] [PubMed] [Google Scholar]
- 164.Hoekenga O.A., Maron L.G., Pineros M.A., Cancado G.M.A., Shaff J., Kobayashi Y., Ryan P.R., Dong B., Delhaize E., Sasaki T., et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mumm P., Imes D., Martinoia E., Al-Rasheid K.A.S., Geiger D., Marten I., Hedrich R. C-terminus-mediated voltage gating of arabidopsis guard cell anion channel QUAC1. Mol. Plant. 2013;6:1550–1563. doi: 10.1093/mp/sst008. [DOI] [PubMed] [Google Scholar]
- 166.Kovermann P., Meyer S., Hörtensteiner S., Picco C., Scholz-Starke J., Ravera S., Lee Y., Martinoia E. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007;52:1169–1180. doi: 10.1111/j.1365-313X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- 167.Dreyer I., Gomez-Porras J.L., Riaño-Pachón D.M., Hedrich R., Geiger D. Molecular Evolution of Slow and Quick Anion Channels (SLACs and QUACs/ALMTs) Front. Plant Sci. 2012;3:1–12. doi: 10.3389/fpls.2012.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eisenach C., De Angeli A. Ion Transport at the Vacuole during Stomatal Movements. Plant Physiol. 2017;174:520–530. doi: 10.1104/pp.17.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Misra B.B., Acharya B.R., Granot D., Assmann S.M., Chen S. The guard cell metabolome: functions in stomatal movement and global food security. Front. Plant Sci. 2015;6:1–13. doi: 10.3389/fpls.2015.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Araújo W.L., Fernie A.R., Nunes-Nesi A. Control of stomatal aperture: A renaissance of the old guard. Plant Signal. Behav. 2011;6:1305–1311. doi: 10.4161/psb.6.9.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van Kirk C.A., Raschke K. Release of malate from epidermal strips during stomatal closure. Plant Physiol. 1978;61:474–475. doi: 10.1104/pp.61.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Asai N., Nakajima N., Tamaoki M., Kamada H., Kondo N. Role of malate synthesis mediated by phosphoenolpyruvate carboxylase in guard cells in the regulation of stomatal movement. Plant Cell Physiol. 2000;41:10–15. doi: 10.1093/pcp/41.1.10. [DOI] [PubMed] [Google Scholar]
- 173.Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006;440:484–489. doi: 10.1038/nature04713. [DOI] [PubMed] [Google Scholar]
- 174.De Angeli A., Monachello D., Ephritikhine G., Frachisse J.M., Thomine S., Gambale F., Barbier-Brygoo H. CLC-mediated anion transport in plant cells. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:195–201. doi: 10.1098/rstb.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zifarelli G., Pusch M. CLC transport proteins in plants. FEBS Lett. 2010;584:2122–2127. doi: 10.1016/j.febslet.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 176.Jentsch T.J., Pusch M. CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol. Rev. 2018;98:1493–1590. doi: 10.1152/physrev.00047.2017. [DOI] [PubMed] [Google Scholar]
- 177.Hechenberger M., Schwappach B., Fischer W.N., Frommer W.B., Jentsch T.J., Steinmeyer K. A family of putative chloride channels from Arabidopsis and functional complementation of a yeast strain with a CLC gene disruption. J. Biol. Chem. 1996;271:33632–33638. doi: 10.1074/jbc.271.52.33632. [DOI] [PubMed] [Google Scholar]
- 178.Lv Q., Tang R., Liu H., Gao X., Li Y., Zheng H., Zhang H. Cloning and molecular analyses of the Arabidopsis thaliana chloride channel gene family. Plant Sci. 2009;176:650–661. doi: 10.1016/j.plantsci.2009.02.006. [DOI] [Google Scholar]
- 179.Andres Z., Perez-Hormaeche J., Leidi E.O., Schlucking K., Steinhorst L., McLachlan D.H., Schumacher K., Hetherington A M., Kudla J., Cubero B., et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA. 2014;111:E1806–E1814. doi: 10.1073/pnas.1320421111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Black V.J., Unsworth M.H. Stomatal responses to sulphur dioxide and vapour pressure deficit. J. Exp. Bot. 1980;31:667–677. doi: 10.1093/jxb/31.2.667. [DOI] [Google Scholar]
- 181.Yi H., Liu X., Yi M., Chen G. Dual role of hydrogen peroxide in Arabidopsis guard cells in response to sulfur dioxide. Adv. Toxicol. 2014;2014 doi: 10.1155/2014/407368. [DOI] [Google Scholar]
- 182.Ooi L., Matsuura T., Munemasa S., Murata Y., Katsuhara M., Hirayama T., Mori I.C. The Mechanism of SO2 -Induced Stomatal Closure Differs from O3 and CO2 Responses and Is Mediated by Non-Apoptotic Cell Death in Guard Cells. Plant. Cell Environ. 2018:1–11. doi: 10.1111/pce.13406. [DOI] [PubMed] [Google Scholar]
- 183.Rouached H., Wirtz M., Alary R., Hell R., Arpat A.B., Davidian J.-C., Fourcroy P., Berthomieu P. Differential Regulation of the Expression of Two High-Affinity Sulfate Transporters, SULTR1.1 and SULTR1.2, in Arabidopsis. Plant Physiol. 2008;147:897–911. doi: 10.1104/pp.108.118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Buchner P., Takahashi H., Hawkesford M.J. Plant sulphate transporters: Co-ordination of uptake, intracellular and long-distance transport. J. Exp. Bot. 2004;55:1765–1773. doi: 10.1093/jxb/erh206. [DOI] [PubMed] [Google Scholar]
- 185.Heidenreich T., Wollers S., Mendel R.R., Bittner F. Characterization of the NifS-like domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J. Biol. Chem. 2005;280:4213–4218. doi: 10.1074/jbc.M411195200. [DOI] [PubMed] [Google Scholar]
- 186.Gallardo K., Courty P.-E., Le Signor C., Wipf D., Vernoud V. Sulfate transporters in the plant’s response to drought and salinity: Regulation and possible functions. Front. Plant Sci. 2014;5:1–7. doi: 10.3389/fpls.2014.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]