Table 7.
Antimicrobial activity of muchimangins analogues.
| No. | Structure | a | Antimicrobial Activity (MIC) |
|---|---|---|---|
| 1S |
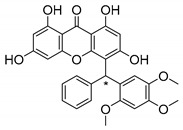
|
± | S. aureus (10.0 µM); B. subtilis (50.0 µM) |
| +2.5 (0.02) | S. aureus (10.0 µM); B. subtilis (50.0 µM) | ||
| −28.0 (0.02) | S. aureus (12.5 µM); B. subtilis (100.0 µM) | ||
| 2S |
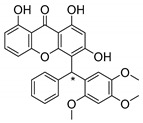
|
± | S. aureus (10.0 µM); B. subtilis (12.5 µM) |
| + | S. aureus (10.0 µM); B. subtilis (10.0 µM) | ||
| - | S. aureus (10.0µM); B. subtilis (12.5 µM) | ||
| 3S |
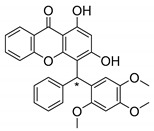
|
± | S. aureus (25.0 µM); B. subtilis (>100.0 µM) |
| + | S. aureus (10.0 µM); B. subtilis (>100.0 µM) | ||
| - | S. aureus (50.0 µM); B. subtilis (>100.0 µM) | ||
| 4S |
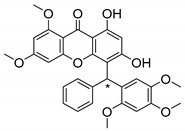
|
± | S. aureus (>100 µM); B. subtilis (>100.0 µM) |
| 5S |
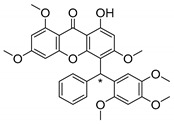
|
± | S. aureus (>100 µM); B. subtilis (>100.0 µM) |
MIC: Minimum inhibitory concentration; a Specific rotation measured in CHCl3; * Stereogenic center; Enantioselectivity is represented by: “±” racemate; “-“ levorotatory; “+” dextrorotatory.
