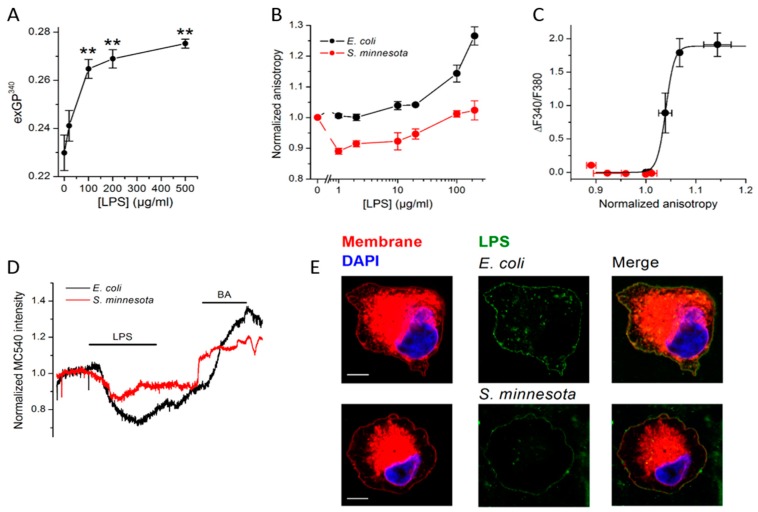
Figure 6.
Effect of LPS insertion on membrane properties. (A) Determination of Laurdan excitation Generalized Polarization (exGP) is a useful tool in describing membrane fluidity as this probe displays a shift in the emission when in contact with ordered or disordered membranes [136,137]. The GP values recorded in one component lipid giant unilamellar vesicles (GUV) are between −0.3 to 0.3 corresponding to the fluid and between 0.5 and 0.6 in gel phase [138]. The exGP values determined in DPPC GUVs indicate membrane rigidification induced by E. coli LPS. (B) Fluorescence anisotropy of membrane sensitive dye diphenylhexatriene (DPH) changes upon alterations of the membrane fluidity. Intercalation of E. coli LPS into CHO-mTRPA1 membranes caused anisotropy increase demonstrating reduction in its fluidity. Contrary, S. minnesota LPS that did not prompt significant changes. (C) Relation between LPS-induced increases in intracellular Ca2+ levels caused by TRPA1 activation and change in DPH fluorescence anisotropy recorded in CHO-TRPA1 cells upon application of E. coli or S. minnesota LPS (0–100 µg/mL), clearly indicted differential effects of both LPS. (D) Merocyanine 540 (MC540), a lipophilic, viscosity probe which fluorescence increases upon binding to membranes in fluid-phase, has been used to further differentiate between effects induced by E. coli or S. minnesota LPS. Benzyl alcohol (BA), a well-described membrane fluidizer [139], has been used after LPS wash out. S. minnesota LPS prompted weaker effects in the plasma membrane mechanical properties that could be attributed to a lower efficiency of its insertion into the cellular membranes (E). Representative Airyscan confocal images of CHO-TRPA1 cells stained with membrane mask (red), DAPI (blue) and treated with Alexa Fluor 488 LPS (green) from E. coli (20 µg/mL top panel) or S. minnesota (20 µg/mL bottom panel). Scale bar, 5 µm. Reproduced with permission from Startek et al. [96].