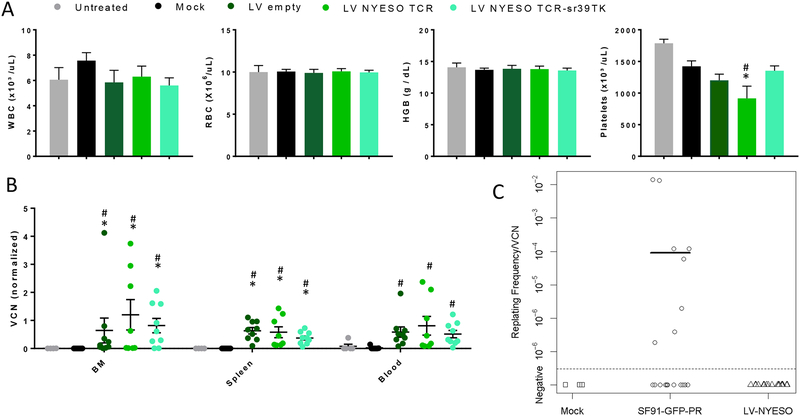
Figure 5. Lack of immunogenicity and genotoxicity of the LV-NY-ESO-1 TCR/sr39TK vector.
Lin- cells transduced with either a LV-NY-ESO-1 TCR/sr39TK, LV-NY-ESO-1 TCR or LV-empty vector were transplanted into myelodepleted HLA-A2/Kb mice. Mice were euthanized at three months after BMT. A. Hematology at 3 months after BMT (n=5–9). WBC, White Blood Cells; RBC, Red Blood Cells; HGB, Hemoglobin. * p<0.05 vs untreated and # p<0.05 vs Mock-transduced. Pair-wise Comparison with Tukey-Kramer. B. Lentivirus VCN in the bone marrow, spleen and blood at three months after BMT. The VCN is normalized with the VCN value of the transplanted cells. Mean ±SEM are plotted (n=6–9). *p<0.05 vs untreated and # p<0.05 vs Mock-transduced. Pair-wise multiple comparison analysis using the Dwass, Steel, Critchlow-Fligner method. C. In vitro immortalization assay. Replating frequency/VCN ratio for mock transduced Lin- cells (n=3), Lin- cells transduced with SF91-eGFP-RRE (n=20) and Lin- cells transduced with the LV-NY-ESO-1 TCR/sr39TK (n=17). Fisher’s exact test (two-sided), p-value = 0.004, and Wilcoxon rank-sum test (two sided), p-value = 0.004, between the SF91-eGFP-RRE transduced group and the LV-NYESO-1 TCR/sr39TK group.