Abstract
Impulsivity, and specific subdomains of inhibitory control and reward sensitivity, are trait-level factors that have been implicated in the onset and maintenance of pediatric obesity and disordered eating, but their associations with real-world eating behavior are unknown. We investigated associations of these trait-level constructs with naturalistic, momentary measures of loss of control (LOC) eating and overeating severity in a heterogeneous sample of youth (n=40), aged 8–14y, with overweight/obesity. Self-report, parent-report, and behavioral data on trait-level impulsivity, reward sensitivity, and inhibitory control, respectively, were collected in the context of a 14-day ecological momentary assessment (EMA) protocol in which participants reported on their eating behavior, mood, hunger, and palatability of foods consumed in real-time. Generalized estimating equations revealed that more perseverative errors on a behavioral measure of visuomotor processing speed and a lower self-reported tendency to act without thinking (at a trend level) were related to greater overall LOC severity. Momentary associations between negative affect and LOC severity were stronger among individuals with greater perseverative errors. Results suggest that trait-level facets of impulsivity may directly influence an individual’s tendency to engage in dysregulated eating behaviors, and may also impact susceptibility to state-level factors associated with occurrence of these behaviors. Momentary interventions for LOC eating may require tailoring to address temperamental factors related to impulsivity and inhibitory control.
Keywords: Loss of control eating, overeating, obesity, impulsivity, reward sensitivity, ecological momentary assessment
Loss of control (LOC) eating, involving a sense that one cannot control what or how much one is eating, and overeating, involving consumption of large amounts of food in a discrete time period (American Psychiatric Association, 2013), are obesogenic eating behaviors that are common among youth with excess weight status (He et al., 2016). These behaviors are associated with physical and psychosocial impairments, independent of obesity, and prospectively predict excess weight gain and onset of full-syndrome eating disorders in youth (Goldschmidt, 2017; Goossens et al., 2007; Morgan et al., 2002; Tanofsky-Kraff et al., 2004). Therefore, both behaviors may be viable intervention targets for preventing eating disorders and obesity and associated negative health outcomes. In particular, interventions delivered in real-time have potential to target antecedents when risk for engaging in dysregulated eating is highest. Yet, very few studies have identified real-time factors associated with LOC and overeating in youth (Hilbert et al., 2009; Ranzenhofer et al., 2014), and none have assessed how these momentary factors interact with trait-level characteristics of individuals who experience these behaviors. Understanding how trait- and state-level factors interact in relation to dysregulated eating could inform development or tailoring of treatments leveraging mobile technologies to intervene in real-time, such as ecological momentary interventions (EMIs; any momentary interventions delivered in the natural environment) or just-in-time-adaptive interventions (JITAIs; momentary interventions with content and/or timing tailored to address the recipient’s current intervention needs).
Inhibitory control and reward sensitivity are two factors that fall under the larger umbrella term of impulsivity, a multi-dimensional construct describing a dispositional tendency to engage in behaviors with limited planning, including those that may be rewarding in the short-term but maladaptive in the long-term (Hammond et al., 2012). Impulsivity and its facets have traditionally been conceptualized from a trait-level perspective. The broader construct of impulsivity, and more specific subdomains of inhibitory control and reward sensitivity, are implicated in the onset and maintenance of obesity and disordered eating across the lifespan (Dohle et al., 2018; Giel et al., 2017; Pearce et al., 2018; Stojek and MacKillop, 2017).
Inhibitory control has been related to dysregulated eating behaviors in both adults and children (Dohle et al., 2018). Inhibitory control deficits may reflect a lack of appropriate restraint over one’s behavior, including a tendency to repeat behaviors that are maladaptive in nature (Bari and Robbins, 2013). Inhibitory control deficits have been related to in vivo eating behavior in adults (Appelhans et al., 2011; Hofmann et al., 2014), but findings are less consistent in children (Adise et al., 2018; Hartmann et al., 2012; Levitan et al., 2015). In addition, it is currently unknown whether and under what conditions (e.g., in the presence of palatable foods, while experiencing negative affect) dispositional inhibitory control relates to children’s real-time, real-world eating behavior.
The construct of reward sensitivity refers to trait-level reactivity and responsivity to rewarding stimuli, including motivation to seek out rewards and tendencies to engage in approach behaviors (Torrubia et al., 2001). Reward sensitivity has been associated with excess body weight and maladaptive eating in children (Carnell et al., 2013; French et al., 2012), although findings have been mixed regarding associations with eating patterns in children (De Decker et al., 2016; Scholten et al., 2014; van den Berg et al., 2011). There are limited data on the role of reward sensitivity in relation to children’s in vivo eating behavior (Rollins et al., 2014). In particular, the association of reward sensitivity with real-world eating patterns has yet to be investigated.
Ecological momentary assessment (EMA) is a method of assessing behavior, cognitions, and emotions in real-time in real-world settings. EMA has numerous advantages over traditional assessment methods relying on retrospective recall and/or summation of experiences over long periods, especially for constructs that are highly variable over time and situations (e.g., emotions, hunger, perceptions of eating behavior). In recent EMA research by our group, we identified real-time food hedonics (perceived palatability of food being ingested), negative affect, and hunger as salient factors related to the occurrence of dysregulated eating in children and adolescents (Goldschmidt et al., 2018b; Haedt-Matt et al., 2018). These findings are consistent with prior EMA research in adults suggesting that 1) the presence of palatable foods is associated with overeating among individuals with a higher body mass index (BMI; Thomas et al., 2011); 2) negative affect is a strong predictor of LOC eating (with or without overeating) across the weight and disordered eating spectrum (Goldschmidt et al., 2014; Haedt-Matt and Keel, 2011b); and 3) hunger is lower prior to eating episodes involving LOC and/or overeating (Goldschmidt et al., 2014; Haedt-Matt and Keel, 2011a). However, the impact of trait-level inhibitory control and reward sensitivity on the momentary associations of food hedonics, negative affect, and hunger with maladaptive eating is unknown.
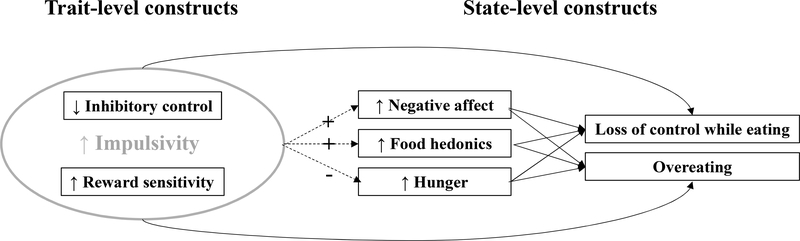
The purpose of the current study was to 1) investigate how trait-level impulsivity facets, including inhibitory control and reward sensitivity, are related to real-world, state-level LOC eating and overeating severity; and 2) assess the extent to which trait-level impulsivity, inhibitory control, and reward sensitivity influence momentary associations of food hedonics, negative affect, and hunger with LOC and overeating severity. We expected that youth higher in trait-level impulsivity and reward sensitivity, and lower in inhibitory control, would demonstrate higher levels of momentary LOC and overeating severity. We further hypothesized that individuals with greater deficiencies in these trait-level constructs would show stronger momentary associations between negative affect and dysregulated eating, with a similar expectation for momentary food hedonics. Finally, because these trait-level factors were hypothesized to promote non-physiological eating behavior (e.g., eating in the absence of hunger), we expected that higher trait-level impulsivity and reward sensitivity, and lower inhibitory control, would attenuate momentary associations between hunger and LOC/overeating severity. See Figure 1 for hypothesized associations among trait- and state-level factors.
Figure 1.
Hypothesized associations among trait- and state-level variables.
Note: Dashed arrows indicated moderation effects; solid arrows indicate main effects. Plus sign indicates strengthening effect; minus sign indicates attenuating effect.
Material and Methods
Participants and Procedures
Participants aged 8–14 years with overweight/obesity [BMI (kg/m2)≥85th percentile for age and sex] were recruited from two academic institutions in Chicago, IL (The University of Chicago Medicine and Illinois Institute of Technology) via community flyers, direct pediatrician referrals, and phone logs from previous studies where the families had consented to be recontacted (Goldschmidt et al., 2018a). Participants were excluded if they 1) had medical conditions (e.g., type 2 diabetes) or were taking medications known to influence weight or appetite, in order to ensure that the sample represented the general population of youth with overweight/obesity; 2) met criteria for an eating disorder other than binge eating disorder; 3) were unable to read and understand English fluently; or 4) were receiving concurrent treatment for overweight/obesity. Caregivers of interested individuals completed a phone screen to assess basic study entry criteria, and eligible participants were invited to attend a baseline study visit, along with a parent or guardian. In total, 92 youth were screened via phone, 44 of whom presented to the research sites for a baseline evaluation, and 40 of whom provided adequate EMA data (e.g., completed at least 1 week of EMA recording) to be included in the current analyses. Participants were 55.0% female (n=22), and self-identified as African-American (62.5%; n=25), Hispanic (17.5%; n=7), non-Hispanic Caucasian (15.0%; n=6), or Asian (2.5%; n=1), reflecting the demographic composition of the study location (not reported, n=1). After providing written informed assent/consent, participants had their height and weight measured and completed interviews and questionnaires assessing eating patterns and psychosocial functioning (see Table 1 for descriptive characteristics). Participants and their caregivers received training on how to complete the EMA protocol, which was administered on a smartphone device. Participants were provided with a loaner smartphone when needed.
Table 1.
Descriptive statistics for trait- and state-level measures
| N | Minimum | Maximum | M | SD | |
|---|---|---|---|---|---|
| Demographic variables | |||||
| z-BMI | 40 | 1.15 | 2.89 | 2.07 | 0.49 |
| Age | 40 | 8.00 | 14.00 | 11.15 | 1.89 |
| State-level measures | |||||
| Loss of control severity1 | 40 | 4.00 | 10.36 | 4.57 | 1.37 |
| Overeating severity1 | 40 | 1.00 | 2.95 | 1.24 | 0.38 |
| Trait-level measures | |||||
| UPPS lack of premeditation | 24 | 11.00 | 30.00 | 21.08 | 6.05 |
| UPPS negative urgency | 24 | 8.00 | 27.00 | 15.38 | 5.22 |
| UPPS sensation seeking | 24 | 10.00 | 30.00 | 21.46 | 5.50 |
| UPPS perseverance | 24 | 11.00 | 29.00 | 20.92 | 4.74 |
| UPPS positive urgency | 23 | 9.00 | 26.00 | 17.26 | 5.21 |
| GMTCT rule break errors | 39 | 0.00 | 11.00 | 1.82 | 2.71 |
| GMTCT perseverative errors | 39 | 0.00 | 1.00 | 0.03 | 0.16 |
| SPSRQ-C impulsivity/fun-seeking | 40 | 1.43 | 4.00 | 2.53 | 0.63 |
| SPSRQ-C drive | 40 | 1.25 | 4.00 | 2.91 | 0.67 |
| SPSRQ-C reward responsivity | 40 | 2.00 | 4.43 | 3.33 | 0.56 |
Note. UPPS-P= Negative Urgency, (lack of) Premeditation, (lack of) Perseverance, Sensation Seeking, and Positive Urgency scales; GMTCT=Groton Maze Timed Chase Task; SPSRQ-C=Sensitivity to Punishment and Sensitivity to Reward Questionnaire for children
State-level variables were aggregated within person, reflecting the average score across all momentary ratings. Loss of control and overeating severity were determined by ratings on a 5-point scale, where 1=“not at all” and 5=“extremely”
Participants were instructed to complete smartphone-based EMA recordings after any type of eating episode (event-contingent); before bedtime (interval-contingent); and at 3–5 semirandom times throughout the day (signal-contingent; Wheeler and Reis, 1991). Signaled prompts occurred every 2–3 hours between 8:00am to 9:00pm on the weekends, and between 7:00–8:00am, 3:00–4:00pm, and 6:00–7:00pm on weekdays so as not to interfere with participants’ school schedules. During all recordings, participants were instructed to describe their current affective, cognitive, and perceptual state, as well as characteristics of any recent eating episode that had not been previously recorded. This combination of signal-, event-, and interval-contingent recordings has been implemented in previous EMA studies of youth with overweight (Hilbert et al., 2009; Ranzenhofer et al., 2014).
A one-day practice period during which adherence was ≥70% of ratings qualified children to initiate the 14-day EMA study period. Consistent with prior EMA studies of eating behavior (e.g., Smyth et al., 2007), these data were not used in statistical analyses to reduce concerns that the immediate adjustment to self-monitoring could induce changes in participants’ behaviors and experiences (Hildebrandt and Latner, 2006), though prior EMA research has demonstrated minimal reactivity effects over longer periods (Stein and Corte, 2003). Participants were contacted by phone by a research assistant after the first day of EMA recording, and every 2–3 days thereafter, to receive feedback regarding their compliance rates and address any questions or concerns regarding assessment procedures.
Upon completing the daily assessment phase, participants returned to the research institution at which they were initially assessed to return loaner smartphones (if applicable), complete a brief, final assessment, and receive their final study incentive. Participants received $50 for the intake assessment; $50 for completion of the 2-week protocol; and up to $50 for daily assessments prorated according to degree of response to random signals ($1 for each response to a total of 50 semi-random signals over the course of the 2-week protocol). Study procedures were approved by The University of Chicago and Illinois Institute of Technology Institutional Review Boards.
Measures
Demographics and Screening Measures.
Demographic data were reported by children and caregivers, and included children’s age, gender, race/ethnicity (White, Black/African-American, Hispanic/Latino, Asian, Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native, or multi-racial/other), current medications, and medical problems. Height and weight were measured in light indoor clothing by a trained research assistant via stadiometer and calibrated digital scale, respectively. Children’s standardized BMI (z-BMI) was calculated using CDC growth charts and accompanying procedures (Kuczmarski et al., 2000). Diagnostic items from the Child Eating Disorder Examination 12.0 (Child EDE; Bryant-Waugh et al., 1996) were used to assess current and lifetime LOC eating and overeating, and rule out other eating disorders. The Child EDE is a semi-structured, interviewer-based instrument adapted from the well-validated adult EDE, with modifications including the use of simpler language appropriate for a younger audience. Assessors completed several hours of Child EDE training with experienced assessors, and conducted at least two interviews to establish reliability with a Child EDE expert before conducting interviews independently. Bi-monthly meetings were held to resolve coding ambiguities and prevent rater drift. The Child EDE has adequate reliability and validity in youth prone to excess weight status (Decaluwe and Braet, 2004), including samples with adequate representation by African-American youth (Tanofsky-Kraff et al., 2004).
Trait-Level Measures.
Impulsivity was assessed at the baseline evaluation via the Negative Urgency, (lack of) Premeditation, (lack of) Perseverance, Sensation Seeking, and Positive Urgency (UPPS-P) scales (Whiteside and Lynam, 2001). The child-report version has demonstrated adequate reliability and validity (Zapolski and Smith, 2013; Zapolski et al., 2010), although psychometric data are based on predominantly White samples. This measure was introduced six months after enrollment began, once it was determined that inclusion of additional baseline measures would not be prohibitively burdensome to participants. Thus data were available for only a subsample of participants. The Groton Maze Timed Chase Task (GMTCT), a computerized measure of visuomotor processing speed, was administered at the final assessment as an objective measure of inhibitory control. The task involves chasing a visual target while following a series of rules (e.g., no skipping tiles, return to the last correct tile after an incorrect move). Perseverative and rule-break errors are considered indices of performance and error monitoring, as they are sensitive to one’s ability to follow rules, correctly respond to feedback, and inhibit pre-potent responding during the task (Pietrzak et al., 2008). Avoiding rule-breaking errors on behavioral tasks through performance monitoring is generally considered to reflect better response inhibition (e.g., Baughman and Cooper, 2007). Reward sensitivity was assessed at the baseline evaluation via the Sensitivity to Punishment and Sensitivity to Reward Questionnaire for children (SPSRQ-C; Colder and O’Connor, 2004), a 33-item parent-report scale. The measure generates a Punishment Sensitivity scale and three Reward Sensitivity scales: Reward Responsivity, Impulsivity/Fun-Seeking, and Drive. Given the study aims, only the Reward Sensitivity scales were included in analyses. The SPSRQ-C is a psychometrically sound alternative to child-report measures of reward sensitivity (Colder and O’Connor, 2004; Luman et al., 2012) which has been validated in racially diverse samples of youth (Colder et al., 2011); only a parent-report measure was included to minimize participant burden.
EMA Measures.
At event-contingent recordings, participants reported on the type of eating episode they experienced (meal, snack, or binge, which were self-determined) as well as several contextual, inter-, and intra-personal features of the episode. Ratings for overeating (“To what extent do you feel that you overate?”) and LOC (“While you were eating ...did you feel a sense of loss of control? ...did you feel that you could not stop eating once you had started? ...did you feel like you could not resist eating? ...did you feel like a car without brakes, you just kept eating and eating?”) were made on a 1- to 5-point Likert-type scale (1=“no, not at all,” and 5=“yes, extremely”). The four items assessing LOC were summed to form a total score (range=4–20) based on their high internal consistency (α=.91). Physiological features included current hunger levels (“Please rate how much you agree with the following statement: I am hungry”), which was rated on a 1- to 5-point Likert-type scale (1=“disagree strongly,” and 5=“agree strongly”), and food hedonics (“On a scale of 1 (terrible) to 10 (the best thing you have ever tasted) how good did the food you ate taste?”). The Positive and Negative Affect Schedule (PANAS; Watson et al., 1988) was used to assess negative mood state. The PANAS is a brief, reliable, and valid measure (Laurent et al., 1999) that has been used in several EMA studies (Engel et al., 2013; Smyth et al., 2007), including studies involving children (Hilbert et al., 2009; Ranzenhofer et al., 2014). Each negative affect item (e.g., afraid, upset) was rated on a 5-point scale (“1”=“Not at all”; “5”=“Extremely”) and summed to form a composite negative affect scale (range=0–50).
Statistical Analyses
Data from eating episodes were limited to recordings occurring within 1 hour of the eating episode. Of the 40 participants who provided adequate EMA data, 38 completed the GMTCT, 24 completed the UPPS-P, and 39 completed the SPSRQ-C. Participants who did not complete a given study measure were excluded from analyses involving those measures, but were included in analyses involving other measures. To examine how trait measures of impulsivity, inhibitory control, and reward sensitivity were related to EMA LOC and overeating severity ratings, separate generalized estimating equations (GEEs) were estimated using a gamma link function to account for skewed distributions of dependent variables and an AR1 serial autocorrelation to account for the dependence within the nested data. Scores from self-/ parent-report measures (i.e., UPPS-P and SPSRQ-C subscales) and task indices (i.e., GMTCT perseverative and rule break errors) were included as independent variables, and EMA variables (i.e., LOC and overeating severity ratings) were included as dependent variables. UPPS-P and SPSRQ-C subscales were entered as simultaneous predictors in separate models; each task index was examined at the univariate level. All trait-level measures were grand-mean centered.
We next evaluated the extent to which these trait-level measures moderated momentary (within-person) associations of negative affect, food hedonics, and hunger with LOC and overeating severity. Moderators were limited to those that demonstrated significant main effects on LOC or overeating severity. EMA measures of negative affect and food hedonics were assessed concurrent to the eating episode, whereas hunger ratings were lagged from the previous signal (within the same day). Separate GEE models were conducted for each EMA predictor (i.e., negative affect, food hedonics, and hunger) of LOC and overeating severity, and in each of the models, the trait measure (i.e., UPPS-P, SPSRQ-C, or GMTCT score) was included as a moderator of these relationships. Each GEE model included person-mean centered and grand-mean centered effects of the EMA predictor, well as main effect of the trait measure, which was grand-mean centered. Lastly, each model included a two-way interaction term between the person-mean centered EMA predictor and the grand-mean centered trait measure, as the present study was focused on individual differences in momentary relationships. Analyses were conducted using SPSS version 25. Alpha was set at .01 to account for multiple comparisons.
Results
A total of 1,656 EMA recordings were available for analysis, of which 471 were eating episodes reported within the last hour. The overall sample completed an average of 13.83 (SD = 1.74) days of EMA recordings during the 14-day study, with a mean of 3.01 (SD = 1.37) total recordings per day across recording types, out of a maximum of 3–5 signal-contingent recordings (depending on weekdays versus weekends) and 1 interval-contingent recordings per day (range = 0.86–7.43; see Goldschmidt et al., 2018b for further details). There was no daily maximum for event-contingent recordings, as participants were instructed to complete these following eating episodes throughout the day and the number of episodes varied by participant.
LOC eating and overeating severity.
Table 2 displays effects of trait-level measures on EMA-measured LOC and overeating ratings. With respect to LOC severity, there was a main effect of GMTCT perseverative errors (B=.26, p<.001) and a trend-level effect of UPPS-P lack of premeditation (B=−.04, p=.012), such that a higher number of perseverative errors and lower scores on the lack of premeditation subscale were related to greater overall LOC severity. No effects were found for other UPPS-P, SPSRQ-C, or GMTCT indices on LOC or overeating severity.
Table 2.
Effects of trait-level impulsivity, inhibitory control, and reward sensitivity measures on momentary loss of control eating and overeating severity
| Loss of control eating severity | Overeating severity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | B | SE | 95% Confidence Interval | Wald χ2 (df=1) | p-value | B | SE | 95% Confidence Interval | χ2 (df=1) | p-value | ||
| Lower | Upper | Lower | Upper | |||||||||
| GMTCT perseverative errors | ||||||||||||
| Intercept | 1.54 | 0.06 | 1.42 | 1.65 | 690.80 | <0.001 | 0.24 | 0.06 | 0.12 | 0.37 | 14.21 | <0.001 |
| GMTCT perseverative errors | 0.26 | 0.06 | 0.15 | 0.38 | 19.46 | <0.001 | −0.05 | 0.07 | −0.18 | 0.08 | 0.59 | 0.442 |
| GMTCT rule break errors | ||||||||||||
| Intercept | 1.55 | 0.06 | 1.43 | 1.66 | 714.51 | <0.001 | 0.24 | 0.06 | 0.12 | 0.36 | 16.27 | <0.001 |
| GMTCT rule break errors | 0.01 | 0.02 | −0.04 | 0.06 | 0.19 | 0.666 | 0.03 | 0.02 | −0.01 | 0.08 | 1.98 | 0.159 |
| UPPS-P | ||||||||||||
| Intercept | 1.56 | 0.06 | 1.43 | 1.69 | 588.30 | <0.001 | 0.19 | 0.07 | 0.05 | 0.34 | 6.82 | 0.009 |
| UPPS-P premeditation | −0.04 | 0.02 | −0.08 | −0.01 | 6.28 | 0.012 | −0.04 | 0.02 | −0.09 | 0.00 | 4.37 | 0.037 |
| UPPS-P negative urgency | −0.01 | 0.02 | −0.05 | 0.04 | 0.13 | 0.718 | <0.01 | 0.03 | −0.06 | 0.05 | 0.01 | 0.935 |
| UPPS-P sensation seeking | −0.01 | 0.01 | −0.03 | 0.01 | 1.29 | 0.255 | <0.01 | 0.01 | −0.02 | 0.03 | 0.05 | 0.816 |
| UPPS-P perseverance | 0.06 | 0.03 | <0.01 | 0.12 | 3.84 | 0.050 | 0.06 | 0.04 | −0.01 | 0.14 | 2.73 | 0.099 |
| UPPS-P positive urgency | 0.02 | 0.03 | −0.03 | 0.07 | 0.70 | 0.401 | 0.01 | 0.03 | −0.05 | 0.08 | 0.16 | 0.693 |
| SPSRQ-C | ||||||||||||
| Intercept | 1.53 | 0.05 | 1.44 | 1.62 | 1038.36 | <0.001 | 0.23 | 0.05 | 0.12 | 0.34 | 17.85 | <0.001 |
| SPSRQ-C impulsivity/fun- seeking | 0.07 | 0.06 | −0.05 | 0.19 | 1.25 | 0.264 | 0.09 | 0.09 | −0.10 | 0.27 | 0.87 | 0.352 |
| SPSRQ-C drive | 0.05 | 0.08 | −0.11 | 0.20 | 0.39 | 0.530 | 0.07 | 0.08 | −0.09 | 0.24 | 0.80 | 0.371 |
| SPSRQ-C reward responsivity | 0.11 | 0.08 | −0.05 | 0.27 | 1.71 | 0.190 | 0.03 | 0.10 | −0.16 | 0.22 | 0.07 | 0.786 |
Note. GMTCT= Groton Maze Timed Chase Task; UPPS-P= Negative Urgency, (lack of) Premeditation, (lack of) Perseverance, Sensation Seeking, and Positive Urgency scales; SPSRQ-C=Sensitivity to Punishment and Sensitivity to Reward Questionnaire for children
Moderation analyses.
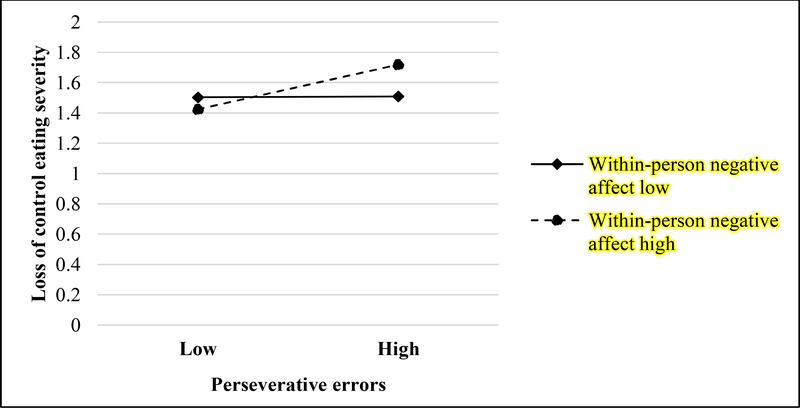
In addition to main effects of GMTCT perseverative errors, momentary (within-person) negative affect, and momentary (within-person) food hedonics on LOC severity, there was a significant interaction between GMTCT perseverative errors and momentary negative affect (B=.14, p<.001), such that the momentary relationship between negative affect and LOC severity was stronger among individuals who evidenced greater perseverative errors (Figure 1 and Table 3). No moderating effect of UPPS-P lack of premeditation was found.
Table 3.
Loss of control severity moderation analyses
| B | SE | 95% Confidence Interval | Wald χ2 (df=1) | p-value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Intercept | 1.54 | 0.06 | 1.43 | 1.65 | 724.90 | <0.001 |
| GMTCT perseverative errors | 0.34 | 0.06 | 0.23 | 0.45 | 36.52 | <0.001 |
| GMC negative affect | 0.03 | 0.02 | −0.01 | 0.08 | 2.08 | 0.150 |
| PMC negative affect | 0.01 | <0.01 | <0.01 | 0.02 | 5.17 | 0.023 |
| GMTCT perseverative errors x PMC negative affect | 0.14 | <0.01 | 0.13 | 0.15 | 869.92 | <0.001 |
| Intercept | 1.54 | 0.06 | 1.42 | 1.65 | 709.19 | <0.001 |
| GMTCT perseverative errors | 0.22 | 0.07 | 0.08 | 0.35 | 10.06 | 0.002 |
| GMC food hedonics | −0.02 | 0.02 | −0.06 | 0.03 | 0.54 | 0.464 |
| PMC food hedonics | 0.01 | <0.01 | <0.01 | 0.01 | 6.82 | 0.009 |
| GMTCT perseverative errors x PMC food hedonics | <0.01 | <0.01 | <0.01 | 0.01 | 1.51 | 0.220 |
| Intercept | 1.52 | 0.04 | 1.43 | 1.61 | 1173.89 | <0.001 |
| GMTCT perseverative errors | 0.37 | 0.04 | 0.29 | 0.44 | 91.74 | <0.001 |
| GMC hunger | 0.18 | 0.10 | −0.02 | 0.38 | 3.19 | 0.074 |
| PMC hunger | 0.02 | 0.02 | −0.03 | 0.06 | 0.50 | 0.477 |
| GMTCT perseverative errors x PMC hunger | 0.03 | 0.02 | −0.02 | 0.07 | 1.46 | 0.227 |
| Intercept | 1.62 | 0.09 | 1.46 | 1.79 | 364.22 | <0.001 |
| UPPS-P lack of premeditation | −0.01 | 0.01 | −0.04 | 0.02 | 0.70 | 0.404 |
| GMC negative affect | 0.04 | 0.04 | −0.05 | 0.13 | 0.83 | 0.361 |
| PMC negative affect | 0.01 | 0.01 | −0.01 | 0.03 | 1.13 | 0.288 |
| UPPS-P lack of premeditation x PMC negative affect | <0.01 | <0.01 | −0.01 | <0.01 | 0.50 | 0.482 |
| Intercept | 1.62 | 0.08 | 1.46 | 1.78 | 394.21 | <0.001 |
| UPPS-P lack of premeditation | −0.01 | 0.02 | −0.04 | 0.02 | 0.24 | 0.621 |
| GMC food hedonics | −0.03 | 0.03 | −0.08 | 0.02 | 1.31 | 0.253 |
| PMC food hedonics | <0.01 | <0.01 | <0.01 | 0.02 | 6.77 | 0.009 |
| UPPS-P lack of premeditation x PMC food hedonics | <0.01 | <0.01 | <0.01 | <0.01 | 0.08 | 0.772 |
| Intercept | 1.59 | 0.05 | 1.49 | 1.70 | 894.75 | <0.001 |
| UPPS-P lack of premeditation | −0.01 | 0.01 | −0.04 | 0.01 | 1.94 | 0.163 |
| GMC hunger | 0.25 | 0.11 | 0.04 | 0.47 | 5.64 | 0.018 |
| PMC hunger | <0.01 | 0.01 | −0.02 | 0.01 | 0.03 | 0.871 |
| UPPS-P lack of premeditation x PMC hunger | <0.01 | <0.01 | −0.01 | 0.01 | <0.01 | 0.967 |
Note. GMTCT= Groton Maze Timed Chase Task; UPPS-P=Negative Urgency, (lack of) Premeditation, (lack of) Perseverance, Sensation Seeking, and Positive Urgency scales; GMC=Grand-mean centered; PMC=Person-mean centered.
Discussion
The current study examined associations of trait-level impulsivity constructs, including inhibitory control and reward sensitivity, with real-world, real-time LOC eating and overeating severity among children and adolescents with overweight/obesity. We found that more perseverative errors on a behavioral measure of visuomotor processing (poorer inhibitory control) and a lower self-reported tendency to act without thinking (lower impulsivity) were related to greater overall LOC severity (the latter at a trend level). Moderation analyses revealed that the momentary relationship between negative affect and LOC severity was stronger among individuals with poorer inhibitory control. Taken together, results suggest that impulsivity and inhibitory control can directly influence an individual’s tendency to engage in dysregulated eating behaviors, and the latter may also impact vulnerability to state-level factors associated with occurrence of these behaviors (i.e., negative affect).
Poorer inhibitory control has been generally linked to LOC and binge eating in several cross-sectional studies of adults and youth (Kittel et al., 2017; Manasse et al., 2015; Manasse et al., 2016; Van Malderen et al., 2018). In the current study, we demonstrated for the first time that such deficits also have direct relevance to the severity of LOC eating in real-time, particularly in the context of greater negative affect. Indeed, poorer inhibitory control may reduce one’s ability to adaptively respond to negative emotions, prompting the use of maladaptive eating behaviors to regulate mood (Eisenberg et al., 2010). Future research should investigate the moderating effects of dispositional inhibitory control on momentary interventions targeting affect and eating behavior in youth.
Contrary to expectations, we found that lower impulsivity was related to greater LOC severity. Although unexpected, this finding may reflect that for some youth, LOC occurs with some degree of premeditation, consistent with conceptualizations of binge eating that suggest some episodes are planned (Pearson et al., 2016). It is also possible that youth who tend to be better at (or place more value on) planning may also have different thresholds for perceiving eating episodes as out of control (e.g., any instance of unplanned eating may be perceived as an LOC episode). Alternatively, this finding may be related to differing perceptions of eating upon initiation versus persistence of eating episodes. That is, lower impulsivity may be related to continuation of eating as reflected in LOC arising during the course of an eating episode, rather than upon commencement of eating. Since the current methods did not allow for distinctions between starting and continuing eating episodes, further research clarifying the nature of this association is warranted.
The current study had several strengths. These included the heterogeneous, community-based sample; the use of self-report, parent-report, and behavioral performance measures (although not all sources of measurement were available for each construct), many of which were validated in samples with adequate racial/ethnic minority representation, marking them as appropriate in this predominantly African-American sample; and the use of EMA to capture real-time, real-world eating- and mood-related factors. Nevertheless, there were several limitations worth noting. First, the sample size was modest (especially for the UPPS-P, which was included after recruitment began and thus was available for only 60% of participants) and included children and younger adolescents, whose eating behaviors may be less entrenched and more constrained by external influences (e.g., parental control) than that of older adolescents. The age range of the sample could have contributed to the modest compliance with EMA recordings, although compliance rates in the current study were somewhat lower than those reported in prior studies of youth (Wen et al., 2017); thus, other sources of bias could have affected response rates (e.g., amount of data requested during recordings; unwillingness to report certain types of eating behaviors), which should be taken into consideration in future studies. Second, LOC and overeating were assessed via self-report without objective corroboration, making it unclear if these constructs map on to empirically-supported conceptualizations (American Psychiatric Association, 2013). Relatedly, reward responsivity was assessed via parent-report only, precluding understanding of how self-perceived reward responsiveness relates to momentary eating behavior. Third, the assessment of inhibitory control was limited to measures of perseveration, and did not address other potentially relevant constructs such as planning, effortful control, and delay of gratification. Therefore, future research should include a broader representation of self-regulation constructs. Finally, given that the study was observational in nature, it was not possible to infer causality of the associations, which will be an important consideration in designing future interventions.
In summary, the current study provides novel information about the extent to which trait-level impulsivity factors are related to one’s susceptibility to engage in maladaptive eating behavior in real-time, particularly in the context of mood-related cues that may be associated with the occurrence of these behaviors (i.e., negative affect). Momentary interventions (e.g., EMIs, JITAIs) for LOC eating in youth may need to be tailored to address specific temperamental factors related to impulsivity in order to improve their relevance and efficacy.
Figure 2.
Interactive effects of trait-level Groton Maze Timed Chase Task perseverative errors and momentary negative affect on momentary loss of control eating severity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adise S, et al. , 2018. Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite 128, 167–179. 10.1016/j.appet.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th edition, Washington, D.C. [Google Scholar]
- Appelhans BM, et al. , 2011. Inhibiting food reward: Delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity 19, 2175–2182. 10.1038/oby.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW, 2013. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol 108, 44–79. 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Baughman FD, Cooper RP, 2007. Inhibition and young children’s performance on the Tower of London task. Cognitive Systems Research 8, 216–226. 10.1016/j.cogsys.2007.06.004. [DOI] [Google Scholar]
- Bryant-Waugh RJ, et al. , 1996. The use of the Eating Disorder Examination with children: A pilot study. Int. J. Eat. Disord 19, 391–397. . [DOI] [PubMed] [Google Scholar]
- Carnell S, et al. , 2013. Appetitive traits from infancy to adolescence: Using behavioral and neural measures to investigate obesity risk. Physiol. Behav 121, 79–88. 10.1016/j.physbeh.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, O’Connor RM, 2004. Gray’s Reinforcement Sensitivity Model and Child Psychopathology: Laboratory and Questionnaire Assessment of the BAS and BIS. J. Abnorm. Child Psychol 32, 435–451. 10.1023/B:JACP.0000030296.54122.b6. [DOI] [PubMed] [Google Scholar]
- Colder CR, et al. , 2011. Revised Reinforcement Sensitivity Theory and Laboratory Assessment of BIS and BAS in Children. Journal of research in personality 45, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Decker A, et al. , 2016. Associations of reward sensitivity with food consumption, activity pattern, and BMI in children. Appetite 100, 189–196. 10.1016/j.appet.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Decaluwe V, Braet C, 2004. Assessment of eating disorder psychopathology in obese children and adolescents: Interview versus self-report questionnaire. Behav. Res. Ther 42, 799–811. doi: 10.1016/j.brat.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Dohle S, et al. , 2018. Executive functions and the self-regulation of eating behavior: A review. Appetite 124, 4–9. 10.1016/j.appet.2017.05.041. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, et al. , 2010. Emotion-Related Self-Regulation and Its Relation to Children’s Maladjustment. Annu. Rev. Clin. Psychol 6, 495–525. 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SG, et al. , 2013. The role of affect in the maintenance of anorexia nervosa: Evidence from a naturalistic assessment of momentary behaviors and emotion. J. Abnorm. Psychol 122, 709–719. 10.1037/a0034010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SA, et al. , 2012. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite 59, 541–549. 10.1016/j.appet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giel KE, et al. , 2017. Food-Related Impulsivity in Obesity and Binge Eating Disorder-A Systematic Update of the Evidence. Nutrients 9. 10.3390/nu9111170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, 2017. Are loss of control while eating and overeating valid constructs? A critical review of the literature. Obes. Rev 18, 412–449. 10.1111/obr.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, et al. , 2014. Ecological momentary assessment of eating episodes in obese adults. Psychosom. Med 76, 747–752. 10.1097/PSY.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, et al. , 2018a. Executive functioning in a racially diverse sample of children who are overweight and at risk for eating disorders. Appetite 124, 43–49. 10.1016/j.appet.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, et al. , 2018b. Ecological momentary assessment of maladaptive eating in children and adolescents with overweight or obesity. Int. J. Eat. Disord 51, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, et al. , 2007. Loss of control over eating in obese youngsters. Behav. Res. Ther 45, 1–9. 10.1016/j.brat.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Haedt-Matt AA, et al. , 2018. Naturalistic assessment of negative affect and hunger related to overeating, loss of control eating and binge eating in overweight youth, Paper presented at the International Conference on Eating Disorders Chicago, IL. [Google Scholar]
- Haedt-Matt AA, Keel PK, 2011a. Hunger and binge eating: A meta-analysis of studies using ecological momentary assessment. Int. J. Eat. Disord 44, 573–578. 10.1002/eat.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, Keel PK, 2011b. Revisiting the affect regulation model of binge eating: A meta-analysis of studies using ecological momentary assessment. Psychol. Bull 137, 660–681. 10.1037/a0023660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond CJ, et al. , 2012. Development of impulse control, inhibition, and self-regulatory behaviors in normative populations across the lifespan, in: Grant JE, Potenza MN (Eds.), The Oxford Handbook of Impulse Control Disorders. Oxford University Press, New York, NY, pp. 232–244. [Google Scholar]
- Hartmann AS, et al. , 2012. Laboratory snack food intake, negative mood, and impulsivity in youth with ADHD symptoms and episodes of loss of control eating. Where is the missing link? Appetite 58, 672–678. 10.1007/s40519-013-0004-4. [DOI] [PubMed] [Google Scholar]
- He J, et al. , 2016. Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis. Int. J. Eat. Disord 10.1002/eat.22661. [DOI] [PubMed] [Google Scholar]
- Hilbert A, et al. , 2009. Loss of control eating and psychological maintenance in children: An ecological momentary assessment study. Behav. Res. Ther 47, 26–33. 10.1016/j.brat.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Latner J, 2006. Effect of self-monitoring on binge eating: Treatment response or ‘binge drift’? Eur. Eat. Disord. Rev 14, 17–22. 10.1002/erv.667. [DOI] [Google Scholar]
- Hofmann W, et al. , 2014. Dieting and the self-control of eating in everyday environments: an experience sampling study. Br J Health Psychol 19, 523–539. 10.1111/bjhp.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel R, et al. , 2017. Executive functions in adolescents with binge-eating disorder and obesity. Int. J. Eat. Disord 10.1002/eat.22714. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, et al. , 2000. CDC growth charts: United States. Adv. Data 314, 1–27. [PubMed] [Google Scholar]
- Lattimore P, Mead BR, 2015. See it, grab it, or STOP! Relationships between trait impulsivity, attentional bias for pictorial food cues and associated response inhibition following in-vivo food cue exposure. Appetite 90, 248–253. 10.1016/j.appet.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Laurent J, et al. , 1999. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychol. Assess 11, 326–338. 10.1037/1040-3590.11.3.326. [DOI] [Google Scholar]
- Levitan RD, et al. , 2015. Gender differences in the association between stop-signal reaction times, body mass indices and/or spontaneous food intake in pre-school children: an early model of compromised inhibitory control and obesity. Int. J. Obes 39, 614–619. 10.1038/ijo.2014.207. [DOI] [PubMed] [Google Scholar]
- Luman M, et al. , 2012. Reward and punishment sensitivity in children with ADHD: validating the Sensitivity to Punishment and Sensitivity to Reward Questionnaire for children (SPSRQ-C). J. Abnorm. Child Psychol 40, 145–157. 10.1007/s10802-011-9547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, et al. , 2015. Do executive functioning deficits underpin binge eating disorder? A comparison of overweight women with and without binge eating pathology. Int. J. Eat. Disord 48, 677–683. 10.1002/eat.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasse SM, et al. , 2016. Slowing down and taking a second look: Inhibitory deficits associated with binge eating are not food-specific. Appetite 96, 555–559. 10.1016/j.appet.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CM, et al. , 2002. Loss of control over eating, adiposity, and psychopathology in overweight children. Int. J. Eat. Disord 31, 430–441. 10.1002/eat.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce AL, et al. , 2018. Executive and Reward-Related Function in Pediatric Obesity: A Meta-Analysis. Child. Obes 10.1089/chi.2017.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, et al. , 2016. Investigating the reinforcing value of binge anticipation. Int J Eat Disord 49, 539–541. 10.1002/eat.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, et al. , 2008. An examination of the construct validity and factor structure of the Groton Maze Learning Test, a new measure of spatial working memory, learning efficiency, and error monitoring. Arch. Clin. Neuropsychol 23, 433–445. 10.1016/j.acn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Ranzenhofer LM, et al. , 2014. Using ecological momentary assessment to examine interpersonal and affective predictors of loss of control eating in adolescent girls. Int. J. Eat. Disord 47, 748–757. 10.1002/eat.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BY, et al. , 2014. Measurement of food reinforcement in preschool children. Associations with food intake, BMI, and reward sensitivity. Appetite 72, 21–27. 10.1016/j.appet.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten EWM, et al. , 2014. Relationship between Impulsivity, Snack Consumption and Children’s Weight. PLOS ONE 9, e88851 10.1371/journal.pone.0088851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, et al. , 2007. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. J. Consult. Clin. Psychol 75, 629–638. 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Stein KF, Corte CM, 2003. Ecologic momentary assessment of eating-disordered behaviors. Int J Eat Disord 34, 349–360. 10.1002/eat.10194. [DOI] [PubMed] [Google Scholar]
- Stojek MMK, MacKillop J, 2017. Relative reinforcing value of food and delayed reward discounting in obesity and disordered eating: A systematic review. Clin. Psychol. Rev 55, 1–11. 10.1016/j.cpr.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, et al. , 2004. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. J. Consult. Clin. Psychol 72, 53–61. 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JG, et al. , 2011. Ecological momentary assessment of obesogenic eating behavior: Combining person-specific and environmental predictors. Obesity 19, 1574–1579. 10.1038/oby.2010.335. [DOI] [PubMed] [Google Scholar]
- Torrubia R, et al. , 2001. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire as a measure of Gray’s anxiety and impulsivity dimensions. Pers. Individ. Dif 31, 837–862. [Google Scholar]
- van den Berg L, et al. , 2011. Association between impulsivity, reward responsiveness and body mass index in children. Int. J. Obes 35, 1301–1307. 10.1038/ijo.2011.116. [DOI] [PubMed] [Google Scholar]
- Van Malderen E, et al. , 2018. Unravelling the association between inhibitory control and loss of control over eating among adolescents. Appetite 125, 401–409. 10.1016/j.appet.2018.02.019. [DOI] [PubMed] [Google Scholar]
- Watson D, et al. , 1988. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wen CKF, et al. , 2017. Compliance With Mobile Ecological Momentary Assessment Protocols in Children and Adolescents: A Systematic Review and Meta-Analysis. Journal of Medical Internet Research 19, e132 10.2196/jmir.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler L, Reis HT, 1991. Self-recording of everyday life events: Origins, types, and uses. J. Pers 59, 339–354. 10.1111/j.1467-6494.1991.tb00252.x. [DOI] [Google Scholar]
- Whiteside SP, Lynam DR, 2001. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers. Individ. Dif 30, 669–689. 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- Zapolski TC, Smith GT, 2013. Comparison of Parent versus Child-Report of Child Impulsivity Traits and Prediction of Outcome Variables. J. Psychopathol. Behav. Assess 35, 301–313. 10.1007/s10862-013-9349-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TC, et al. , 2010. The measurement of dispositions to rash action in children. Assessment 17, 116–125. 10.1177/1073191109351372. [DOI] [PMC free article] [PubMed] [Google Scholar]