Abstract
Background:
While ketamine has been increasingly studied for treatment resistant depression (TRD), the impact of sex differences on treatment outcomes has not been well studied. The objective was to ascertain whether there were differences in response to a single administration of ketamine for TRD between men and women, and between pre- and post-menopausal women.
Methods:
A randomized, double-blind, placebo-controlled trial (N=99; N=50 male; N=49 female) was conducted to investigate the efficacy of intravenous ketamine versus active placebo as augmentation of antidepressant therapy for TRD. Patients were assigned to one of five arms; one-time administration of ketamine of varying doses (i.e., 0.1, 0.2, 0.5, and 1.0 mg/kg), and one group receiving active placebo (intravenous midazolam). A priori-planned analyses were conducted to compare responses between women and men, as well pre- vs. postmenopausal women.
Results:
Analyses demonstrated no significant differences between women and men in terms of treatment response (F(1,80)=0.06, p=0.80). There were no significant differences in the frequency of adverse effects (AEs) reported by those assigned to ketamine treatment groups (p>0.21 for all AEs reported more than once), although women reported more headaches (12% vs. 6%, p=0.30) and nausea (10% vs. 6%, p=0.47). In comparing pre- vs. postmenopausal women, no differences in efficacy were observed (F(1,76)=0.36, p=0.55).
Conclusions:
Results do not support differential efficacy or tolerability of ketamine for the treatment of TRD between women and men, nor based on menopause status among women. However, larger trials with these a priori aims are needed to confirm these results.
Keywords: ketamine, depression, treatment resistant, sex differences, menopause
INTRODUCTION
Ketamine has progressively received increased attention as an expeditiously acting treatment for major depressive disorder (MDD), treatment resistant depression (TRD), and suicidality (Duman et al., 2016; Sanacora et al., 2017; Tundo et al., 2015; Wilkinson et al., 2018). Ketamine is a dissociative anesthetic and an antagonist of N-methyl-d-aspartate receptors (NMDAr) (Duman et al., 2016; Sanacora et al., 2017; Tundo et al., 2015; Wilkinson et al., 2018). A consensus statement around the use of ketamine for the treatment of depression was recently published, detailing the robust and rapid antidepressant effects of ketamine in randomized controlled trials, while underscoring the limitations of the current evidence base (Sanacora et al., 2017). As ketamine use becomes more prevalent and accessible, a better understanding of predictors of patient response and tolerability is needed. Previous studies have suggested that there may be sex differences among men and women in terms of response to monoaminergic antidepressants (Kornstein et al., 2000; Kornstein et al., 2006; Sloan and Kornstein, 2003; Young et al., 2009), and it would be important to know if differences occurred with ketamine use. Also, it is possible that treatment responses may differ among women according to menopause status, with premenopausal women responding differently to postmenopausal women, and that perimenopausal women may also have different treatment responses. Previously in the assessment of treatment responses to SSRI and SNRI therapy in two large studies, Kornstein et al. did not find differences among women based on menopause status nor exogenous sex hormone treatment (Kornstein et al., 2014; Kornstein et al., 2013). However, in a study in which patients received either the tricyclic antidepressant imipramine or the SSRI sertraline, women responded better to sertraline than to imipramine overall, with premenopausal women showing a significantly more robust response to sertraline than imipramine, a difference not found among postmenopausal women (8). In the same study, men were found to have better responses to imipramine than sertraline, representing a significant sex difference in treatment response.
It is plausible that men and women may respond differently to ketamine treatment, and that women may respond variably based on reproductive lifecycle status, specifically regarding whether they are pre- or postmenopausal. Animal models suggest that the neuropsychiatric sequelae of ketamine may be affected by sex and gonadal steroids. In one study, ketamine was found to induce a schizophrenia-like state in male and diestrous female rats (in a low estradiol phase), while these behaviors were not observed in female rats during the high estrous phase (Celia Moreira Borella et al., 2016), suggesting that a high-estrogen state may dampen a ketamine-related response. Other findings in animal studies have also suggested that sex may influence rates of ketamine metabolism (Guo et al., 2016; Livingston and Waterman, 1977).
In animal models of depression, sex differences in response to ketamine have been inconsistent and challenging to apply to humans. Female rats appear to respond more robustly to ketamine administration in the forced swim test, although effects may last longer in males (Franceschelli et al., 2015). There may be an interaction between estrogen and ketamine, with female rats demonstrating more of an antidepressant-like response to low dose ketamine, not observed among male or ovariectomized female rats. Antidepressant-like effects that are not observed in ovarectomized animals have been observed when physiological replacement of both estrogen and progesterone were provided (Carrier and Kabbaj, 2013; van den Buuse et al., 2015). In another study of repeated ketamine administration, male rats experienced an antidepressant-like effect, while female rats demonstrated behaviors consistent with anxiety and depression (Thelen et al., 2016). Ketamine administration in animals has also led to observations about neurochemical effects differentiated by sex (Sarkar and Kabbaj, 2016; Thelen et al., 2016). Taken together, these studies support the assessment of possible sex differences in response to ketamine in humans and potential roles of estrogen and progesterone in those differences.
In humans, few studies have yet to inform whether ketamine administration might have clinically important sex differences in the treatment of depression or other neuropsychiatric indications. In a pooled analysis of four studies assessing ketamine infusions as a treatment for MDD or bipolar depression, investigators did not find that sex was predictive of depression outcomes (Niciu et al., 2014). However, a meta-analysis that included N=437 patients demonstrated that men appeared to maintain antidepressant responses to ketamine longer than women after a single dose administration (Coyle and Laws, 2015). Regarding tolerability, in a study of ketamine abusers undergoing substance abuse treatment, withdrawal symptoms and subjectively reported cognitive symptoms were more common among females compared to males (Chen et al., 2014). In contrast, in a retrospective study of fifty patients who received ketamine for sedation or pain relief on medical and surgical units, no differences in psychiatric adverse effects were found (Rasmussen, 2014).
This trial of ketamine was conducted as part of a collaboration between the MGH Clinical Trials Network and Institute (CTNI), multiple academic sites, and the National Institute of Mental Health (NIMH), with the goal of assessing rapid onset antidepressant effects of treatment with a range of ketamine doses (Fava et al., in press).
We aimed to assess the impact of sex and female lifecycle status on the short-term antidepressant effects of ketamine infusions. The objective of these a priori analyses were to: 1) assess sex differences in rapid antidepressant response to ketamine treatment between men and women, with the primary outcome in depression response at 24 hours, 2) to assess differences in antidepressant response between pre- and postmenopausal women, and 3) to assess the potential impact of gonadal sex hormones at baseline on the antidepressant response of ketamine in females, to ascertain whether sex hormones may serve as a potential biomarker of response.
METHODS
Overview
These analyses were planned a priori as part of a multi-site treatment study designed to assess the rapid-onset antidepressant effects of ketamine therapy for TRD and to assess the efficacy of differential dosing (Fava et al., in press). In brief, this was a randomized, doubleblind, placebo-controlled study of the acute efficacy of intravenous ketamine or placebo added to ongoing, stable, and adequate antidepressant therapy (ADT) in the treatment of adults with TRD. Subjects were consented, documented with written informed consent forms that were approved by the site IRBs and NIMH Data Safety and Monitoring Board. Patients were randomized to one of five possible arms in a 1:1:1:1:1 fashion: a single dose of ketamine 0.1 mg/kg (n=18), a single dose of ketamine 0.2 mg/kg (n=20), a single dose of ketamine 0.5 mg/kg (n=22), a single dose of ketamine 1.0 mg/kg (n=20), or a single dose of midazolam 0.045 mg/kg (n=19).
Subjects
Male and female outpatients between the ages of 18 and 70 years were eligible for enrollment if they were diagnosed with MDD and were experiencing a major depressive episode (MDE) of at least eight weeks in duration prior to screening as defined by the Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (AP, 2000) (DSM-IV-TR™) criteria (AP, 2000). Eligible subjects were also confirmed to be experiencing TRD during the current MDE, defined as a failure to achieve a satisfactory response to at least two adequate treatment courses of antidepressant therapy with a minimal dose approved for the treatment of MDD and of at least eight weeks’ duration (<50% response). For at least four weeks prior to screening, patients were required to be on stable doses of antidepressants. Remote raters confirmed that patients met inclusion criteria.
For a complete summary of the inclusion/exclusion criteria used in this protocol, please refer to the original study (Fava et al., in press).
Methods and Study Medication
Participants were screened between 7 to 28 days, during which eligibility was determined, and prohibited medications were discontinued. Eligible subjects proceeded to the baseline visit. Participants were stratified by body mass index (BMI) (≤30 and >30), and were block randomized into each of the five arms of the study. N= 99 total subjects were randomly assigned to each of these five arms in a 1:1:1:1:1 fashion; four of these treatment groups received ketamine at different single-dose administration (0.1 mg/kg, 0.2 mg/kg, 0.5 mg/kg, and 1.0 mg/kg respectively), and those in the control group received a single dose of midazolam, the active placebo (0.045 mg/kg).
At the baseline visit (Day 0), subjects received either ketamine or placebo by continuous infusion for 40 minutes. The study drug was administered intravenously via an electronic syringe infusion pump, and subjects were continuously monitored throughout the process.
Assessments
Remote raters blinded to treatment assignment were utilized to maintain study blinding. The primary outcome measure was the change on the 6-item Hamilton Depression Rating Scale (HAM-D6) at 24 hours after treatment (Bech et al., 1981; Bech et al., 2010; Hamilton, 1960; O’Sullivan et al., 1997). This version of the HAM-D was utilized as it has been shown to be more sensitive for the detection of changes with acute treatment than the original 17-item version (Bech et al., 2010). Secondary measures are described in the primary report of the study outcomes (Fava et al., in press). With respect to patient safety, the Columbia Suicide Severity Rating Scale (C-SSRS) (Posner et al., 2007), the Clinician-Administered Dissociative States Scale (CADSS) (Bremner et al., 1998), and the Systematic Assessment for Treatment Emergent Events - Systematic Inquiry (Levine and Schooler, 1986) (SAFTEE-SI) were utilized to measure any emergent suicidal ideation and behavior, as well as adverse events including dissociation during infusion.
The MGH Female Reproductive Lifecycle and Hormones Questionnaire (FRLHQ) (Freeman et al., 2013) was utilized to systematically assess and prospectively document reproductive lifespan status (pre-, peri-, or postmenopausal), use of exogenous hormones, including hormonal birth control and hormone treatment for menopausal symptoms, and menstrual cycle phase.
Laboratory Assessments
Laboratory assessments included hormonal measures collected at Visit 1. For female subjects, this included estradiol, progesterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), Sex hormone-binding globulin (SHBG), testosterone, and free testosterone, and for male subjects, testosterone, free testosterone, SHBG, dehydroepiandrosterone (DHEA), and progesterone.
Analytic Strategy
Analyses focused on Day 1 outcomes, even though ketamine was assessed on days 3, 5, 7, 14, and 30 as well, because previous analyses found that Day 1 differences largely accounted for the 72-hour hypothesized effect of ketamine (cite main outcome paper). Thus, in order to maximize the chances of finding a gender effect in these exploratory analyses, we focused analyses on Day 1.
In order to test if there was a gender difference in the response to ketamine on the HAMD6 on Day 1, we fit a linear mixed effects model where HAMD6 score was the dependent variable, and predictor variables were DAY (Day 0 (pre infusion), Day 1), GENDER (male, female), GROUP (midazolam, ketamine), and their interaction terms. Of interest was the GENDER*DAY*GROUP interaction term, which tests if there was a gender effect on the GROUP*TIME interaction effect, which captures the effect of randomized group on HAMD6 scores. We modeled observations as nested within individuals, and included a random effect for SITE (6 sites). To examine if there was a different effect based on ketamine dosage, we used the same model, but used the 5-level GROUP variable (midazolam, 0.1 mg/kg. 0.2 mg/kg, 0.5 mg/kg, 1.0mg/kg) instead of the 2-level GROUP variable.
To examine gender differences long-term, we also fit a model for HAMD6 scores observed on days 1-30. To this end, we fit a linear mixed effects model where HAMD6 score was the dependent variable, and predictor variables were DAY (1, 3, 5, 7, 14, 30), GENDER (male, female), GROUP (midazolam, ketamine), and BASELINE (i.e., baseline HAMD6 scores, as measured on day 0). Because this model fits a line across follow-up, and does not include Day 0 in the outcome vector, the effect of interest in this model was the GENDER*GROUP interaction effect. If significant, this interaction term would suggest gender differences in HAMD6 group by group during the follow-up phase. In building this model, we first included 2- and 3-way interaction terms for DAY, GENDER, and GROUP, and then removed them one by one if not significant, with the exception of the hypothesized interaction term, GENDER*GROUP. As before, we modeled observations as nested within individuals, and included a random effect for SITE (6 sites). To examine if there was a different effect based on ketamine dosage, we used the same model, but used the 5-level GROUP variable.
Next, we tested if there was a difference between women of different reproductive lifespan status. We used the same models as described for gender differences in Day 1 response to ketamine, except that we limited analyses to women, and instead of using GENDER as a predictor variable, we used MENOPAUSE (pre- vs. post-menopausal). N=3 women were excluded from these analyses, because they were neither pre- nor post-menopausal (i.e., peri-menopausal).
Finally, we used the same models to test for an effect of baseline hormonal levels on response to ketamine. Analyses were restricted to women. Instead of using the categorical predictor MENOPAUSE, we used the continuous lab values as the predictor. We tested 7 hormonal tests (i.e., estradiol, progesterone, FSH, LH, SHBG, Testosterone, and Free T).
RESULTS
Baseline Comparisons
Please see Table 1 for comparisons at baseline between men and women, and between pre- and postmenopausal women. Because the number of perimenopausal women was so small (N=3), these women were excluded from statistical comparisions. On most variables at baseline, men and women were similar, as were pre- and postmenopausal women, with the exception of the expected finding that postmenopausal women being older than premenopausal women.
Table 1.
Baseline Characteristics of Study Sample by Sex and Menopause Stage
| Gender |
Menopausal Stage |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male n=50 | Female n=49 | Pre n=30 | Post n=16 | |||||||
| mean/% | (SD/n) | mean/% | (SD/n) | p | mean/% | (SD/n) | mean/% | (SD/n) | p | |
| Demographics | ||||||||||
| Age | 47.5 | (12.5) | 44.8 | (12.7) | 0.29 | 36.6 | (8.3) | 58.9 | (5.7) | <.0001 |
| Hispanic (% yes) | 2.0 | (1) | 4.1 | (2) | 0.52 | 6.7 | (2) | 0.0 | (0) | 0.42 |
| Race | 0.67 | 0.45 | ||||||||
| White | 92.0 | (46) | 85.7 | (42) | 80.0 | (24.0) | 100.0 | (16.0) | ||
| Asian | 4.0 | (2) | 6.1 | (3) | 6.7 | (2.0) | 0.0 | (0.0) | ||
| Black | 4.0 | (2) | 4.1 | (2) | 6.7 | (2.0) | 0.0 | (0.0) | ||
| Other | 0.0 | (0) | 4.1 | (2) | 6.7 | (2.0) | 0.0 | (0.0) | ||
| Clinical Severity at Baseline | ||||||||||
| HAMD6 | 12.4 | (2.1) | 13.0 | (2.0) | 0.16 | 13.2 | (2.2) | 12.8 | (1.7) | 0.54 |
| Reproductive Lifecycle | ||||||||||
| Menopausal status | ||||||||||
| Premenopausal | 61.2 | (30) | ||||||||
| Perimenopausal | 6.1 | (3) | ||||||||
| Postmenopausal–non-surgical | 26.5 | (13) | ||||||||
| Postmenopausal–surgical | 6.1 | (3) | ||||||||
| Use of hormone therapy (% yes) | 28.6 | (14) | 36.7 | (11) | 18.8 | (3) | 0.21 | |||
| Randomized Group | 0.79 | 0.96 | ||||||||
| ketamine 0.1 mg/kg | 16.0 | (8) | 20.4 | (10) | 20.0 | (6) | 18.8 | (3) | ||
| ketamine 0.2 mg/kg | 22.0 | (11) | 18.4 | (9) | 20.0 | (6) | 18.8 | (3) | ||
| ketamine 0.5 mg/kg | 22.0 | (11) | 22.4 | (11) | 20.0 | (6) | 25.0 | (4) | ||
| ketamine 1.0 mg/kg | 24.0 | (12) | 16.3 | (8) | 20.0 | (6) | 12.5 | (2) | ||
| midazolam 0.045 mg | 16.0 | (8) | 22.4 | (11) | 20.0 | (6) | 25.0 | (4) | ||
Note: N=3 were perimenopausal, and were excluded from menopausal stage analyses
Effect of gender on response to ketamine at Day 1
When testing the 2-group difference (all ketamine groups combined vs. midazolam) (Table 2), the GENDER*DAY*GROUP interaction term was not significant (F(1,180)=0.06, p=0.80). Results were similar when modeling ketamine by its different dosages by using a 5-level GROUP variable (midazolam, 0.1 mg/kg. 0.2 mg/kg, 0.5 mg/kg, 1.0mg/kg). As in the 2-group model, the GENDER*DAY*GROUP interaction term was not significant (F(4,168)=1.04, p=0.39). Unlike in the 2-level GROUP variable model, the GROUP*GENDER effect was significant (F(4,168)=2.89, p=0.02). In examining this non-hypothesized effect further, we noted significant differences by GENDER in the ketamine 0.5 mg/kg and 1.0 mg/kg group, where women in the ketamine 0.5 mg/kg group had higher HAMD6 scores than men at 24 hours (least squares means = 10.5 vs. 8.0), but women in the ketamine 1.0 mg/kg group had lower HAMD6 scores than men (least squares means = 8.3 vs. 10.8). This effect likely captures pre-existing differences in women vs. men.
Table 2.
Type 3 tests of mixed effects model testing for a gender difference on HAMD6 on days 1-30
| 2-group comparison | 5-group comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F | Num DF | Den DF | F Value | Pr > F |
| GROUP | 1 | 180 | 7.25 | 0.01 | 4 | 168 | 3.73 | 0.01 |
| DAY | 1 | 180 | 54.51 | <.0001 | 1 | 168 | 140.00 | <.0001 |
| GENDER | 1 | 180 | 0.24 | 0.63 | 1 | 168 | 0.97 | 0.32 |
| GROUP*DAY | 1 | 180 | 8.00 | 0.01 | 4 | 168 | 4.13 | 0.00 |
| GROUP*GENDER | 1 | 180 | 0.16 | 0.69 | 4 | 168 | 2.89 | 0.02 |
| DAY*GENDER | 1 | 180 | 0.05 | 0.83 | 1 | 168 | 0.00 | 0.98 |
| GROUP*DAY*GENDER | 1 | 180 | 0.06 | 0.80 | 4 | 168 | 1.04 | 0.39 |
In examining HAMD6 scores across Days 1-30 (illustrated in Figure 1), we also did not identify a statistically significant gender difference. Specifically, when testing the 2-group difference (all ketamine groups combined vs. midazolam), none of the interaction terms were significant, so that we removed, in turn, the interaction terms for DAY*GENDER*GROUP (F(5,508)=0.19, p=0.97), DAY*GROUP (F(5,513)=1.53, p=0.18), and DAY*GENDER (F(5,518)=0.47, p=0.80). We kept the hypothesized GENDER*GROUP effect in the model regardless of significance. The results of this reduced model did not identify an overall significant effect of gender on ketamine response, with F(1,523)=0.70, p=0.40 for the GENDER*GROUP interaction effect.
Figure 1.
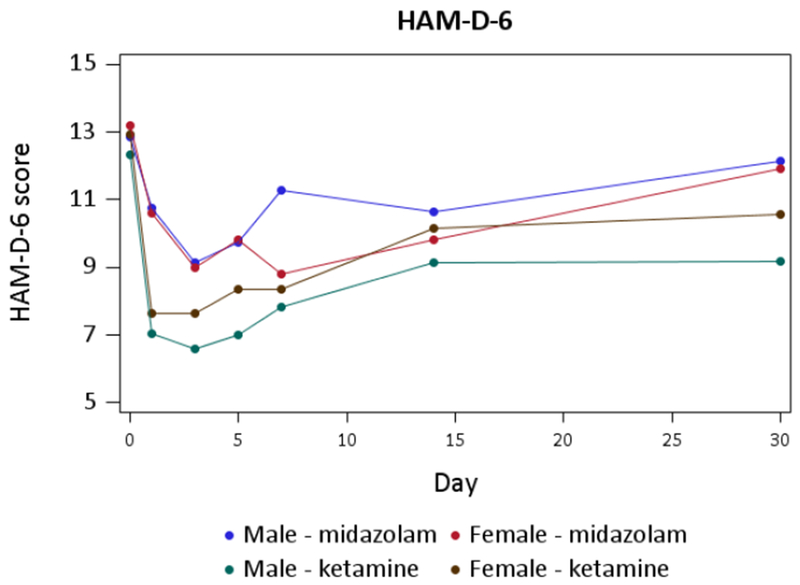
Gender differences in HAMD6 scores across Days 1-30
Results were similar when modeling ketamine by its different dosages by using a 5-level GROUP variable (midazolam, 0.1 mg/kg. 0.2 mg/kg, 0.5 mg/kg, 1.0mg/kg). As in the 2-group model, none of the interaction terms were significant, so that we removed, in turn, the interaction terms for DAY*GENDER*GROUP (F(20,472)=0.71, p=0.81), DAY*GROUP (F(20,492)=1.20, p=0.25), and DAY*GENDER (F(5,512)=0.47, p=0.80). The results of this reduced model also did not identify an overall significant effect of gender on ketamine response, with F(4,517)=1.28, p=0.28 for the GENDER*GROUP interaction effect.
Effect of reproductive lifespan status on response to ketamine
Similar to the gender difference analyses focused on Day 1, when testing the 2-group difference (all ketamine groups combined vs. midazolam), the MENOPAUSE *DAY*GROUP interaction term was not significant (F(1,76)=0.36, p=0.55). When repeating the model using the 5-level GROUP variable, the MENOPAUSE *DAY*GROUP interaction term remained non-significant (F(4,64)=1.03, p=0.40). Similarly to the gender difference analyses, the non-hypothesized GROUP*MENOPAUSE interaction effect was significant (F(4,64)=3.26, p=0.02). In examining this effect further, we noted significant differences by MENOPAUSE in the ketamine 0.2 mg/kg group, where premenopausal women (n=6) had higher HAMD6 scores than postmenopausal women (n=3) (LS means = 12.9 vs. 9.7, respectively), reflecting pre-existing differences prior to randomization.
Effect of baseline hormonal values on response to ketamine
The 3-way interaction term GROUP*DAY*LAB was non-significant for all seven hormonal variables we tested, in both the 2-group (p≤0.21 across the 7 hormonal variables) and 5-group analyses (p≤0.19 across the 7 variables) (Table 3). The main effect for hormonal level was non-significant across all models. Two-way interactions were significant only for the GROUP*LAB interaction in the model involving SHBG values, in both the 2-group (p=0.005) and 5-group comparison (p=0.0046).
Table 3.
Effect of hormonal level on response to ketamine in women (i.e., significance of the GROUP*DAY*LAB effect)
| 2-group comparison | 5-group comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| Hormonal Measure | Num DF | Den DF | F Value | Pr > F | Num DF | Den DF | F Value | Pr > F |
| Female Estradiol | 1 | 66 | 0.14 | 0.71 | 4 | 54 | 1.09 | 0.37 |
| Female Progesterone | 1 | 73 | 0.00 | 0.96 | 4 | 61 | 0.20 | 0.94 |
| Female FSH | 1 | 77 | 1.57 | 0.21 | 4 | 65 | 1.57 | 0.19 |
| Female LH | 1 | 81 | 1.16 | 0.28 | 4 | 69 | 0.73 | 0.57 |
| Female SHBG | 1 | 81 | 0.67 | 0.42 | 4 | 69 | 0.45 | 0.77 |
| Female Testosterone | 1 | 77 | 0.22 | 0.64 | 4 | 65 | 1.10 | 0.36 |
| Female Free T | 1 | 69 | 0.58 | 0.45 | 4 | 57 | 0.99 | 0.42 |
Note: degrees of freedom vary across models for the different hormornal variables, because of missing lab results for some women.
Tolerability
In terms of tolerability, there were also no significant differences between women and men in the frequency of adverse effects (AEs) reported by those assigned to the ketamine groups (p>0.21 for all AEs reported more than once study-wide), although women reported more headaches (12% vs. 6%, p=0.30) and nausea (10% vs. 6%, p=0.47).
DISCUSSION
These findings support that one-time use of ketamine is a similarly effective and tolerable treatment for TRD for both women and men. Specifically, these analyses did not demonstrate significant differences in response to ketamine between the two groups, and also did not discern differences in treatment response between pre- and postmenopausal women. The results of these analyses are consistent with two past studies, which reported that sex did not have a significant effect on depression treatment outcomes (Niciu et al., 2014; Salvadore et al., 2012), as well as with some animal models (Kara et al., 2017). However, they are in contrast with other studies in both humans and animals that suggest sex differences (Carrier and Kabbaj, 2013; Celia Moreira Borella et al., 2016; Coyle and Laws, 2015; Franceschelli et al., 2015; Guo et al., 2016; Sarkar and Kabbaj, 2016; van den Buuse et al., 2015; Wright et al., 2017). Additionally, although sex hormones have been shown to modify ketamine treatment response in some animal studies (Carrier and Kabbaj, 2013; Celia Moreira Borella et al., 2016; van den Buuse et al., 2015; Wright et al., 2017), our current findings did not suggest that baseline hormone levels predicted response in humans.
Although we did not find significant differences between men and women in terms of ketamine tolerability, additional research using larger sample sizes would be required to definitively conclude that this is the case. Several rodent studies have demonstrated potential sex differences in adverse events (Carrier and Kabbaj, 2013; Jevtovic-Todorovic et al., 2001; Thelen et al., 2016), suggesting more adverse effects in females compared to males. In humans, studies have been inconsistent with regard to sex differences in adverse effects to ketamine, both in samples of ketamine abusers and in those who received ketamine for the treatment of MDD, sedation or pain relief (Rasmussen, 2014). Importantly, there may be salient sex differences in some adverse events, such as ketamine-induced hypertension, as in one study women experienced higher maximal diastolic blood pressure changes with ketamine administration than men (Liebe et al., 2017). Ketamine abuse data indicate that female users may have a greater risk of psychiatric comorbidity (Tang et al., 2015), increased withdrawal symptoms, and subjectively reported cognitive symptoms (Chen et al., 2014).
If further research supports sex differences in ketamine treatment efficacy and safety contrary to our findings, it will be important to explore how underlying mechanisms contribute to distinctions. Previous studies have suggested sex differences in ketamine metabolism (Guo et al., 2016; Jevtovic-Todorovic et al., 2001; Livingston and Waterman, 1977). Other mechanisms could relate to sex differences in the function of glutamatergic cells (Gray et al., 2015) or variables of genetic expression (Gray et al., 2015).
Strengths of these analyses include the randomized, placebo-controlled design of the original study, and the a priori collection of detailed information about female participants’ reproductive lifecycle status, i.e., pre-, peri, and postmenopausal status, and collection of hormonal assays at baseline and assessment with treatment response.
However, there are important limitations to these analyses. The study was not powered to assess sex and female reproductive lifespan status as predictors of response or tolerability. Additionally, the study was designed to assess rapid effects of ketamine, with a focus on the response to a single treatment at 24- and 72-hours after infusion. Therefore, we are not able to generalize these results to serial treatment with repeated administration of ketamine, nor longer term outcomes. In addition, due to the sample size, we were not able to take into account the use of hormonal contraceptives and timing of the menstrual cycle, both of which introduce additional variables that may be important in the assessment of the impact of gonadal hormones on response to ketamine.
In the study of ketamine and any treatment for a psychiatric disorder, in order to determine the best treatments for the specific patient sub-groups, variables pertaining to endogenous and exogenous reproductive hormones should be prospectively collected and well documented in clinical trials. Although in this trial we did not see differential outcomes when comparing men and women, and reproductive lifecycle status among women, future studies of ketamine may elucidate predictors of response based on sex, reproductive status, or exogenous hormone use, and it is possible that biomarkers such as gonadal steroids may predict treatment response. Typically in clinical trials for MDD, the focus of collection of reproductive data pertains strictly to the use of contraception, and reproductive lifecycle status is not usually explicitly documented. In addition, phases of the menstrual cycle and precise data regarding exogenous hormonal treatments is not usually well documented in studies of depression. Therefore, precise collection of these variables should be encouraged in future trials. The ability to analyze such variables may assist in honing the ability to determine which patients will respond best to specific treatments.
Acknowledgments
Funding Source: National Institute of Mental Health; NIH-NIMH HHSN271201100006I
Conflict of Interest Statements:
Marlene Freeman:
Over the past three years, Dr. Freeman has received research support from: Takeda, JayMac, and Sage; she has served in advisory boards of: Janssen, Sage, JDS therapeutics, Sunovion, and Takeda; she has served in the Independent Data Safety and Monitoring Committee of Janssen (Johnson& Johnson); she has served as a medical editor for the GOED newsletter.
Dr. Freeman is an employee of Massachusetts General Hospital, and works with the MGH National Pregnancy Registry [Current Registry Sponsors: Teva, Alkermes, Inc. (2016-Present); Otsuka America Pharmaceutical, Inc. (2008-Present); Forest/Actavis (2016-Present), Sunovion Pharmaceuticals, Inc. (2011-Present)]. As an employee of MGH, Dr. Freeman works with the MGH CTNI, which has had research funding from multiple pharmaceutical companies and NIMH.
George I. Papakostas:
Over the past three years, Dr. Papakostas has consulted to: Lundbeck, Sunovion, Brainsway, Pfizer, Boston Pharmaceuticals*, Novartis*, Acadia*, Axsome*, Genomind*, and Mylan*(*on behalf of Massachusetts General Hospital). He has received honoraria from: Lundbeck, Grunbiotics-Mylan, Takeda, Alkermes, Pfizer, Pharma Trade SAS, Asofarma, Sunovion, Brainsway, and Unilab Philippines. He has received research support from Neuralstem Inc and Tal Medical.
Bettina B. Hoeppner:
Dr. Hoeppner does not have any COI to declare for this paper.
Erica Mazzone:
Erica Mazzone does not have any conflicts of interest to report.
Heidi Judge:
Ms Judge does not have any conflicts of interest to report.
Cristina Cusin:
Dr. Cusin receives funding from NIMH (R01MH102279) and has received consulting fees from Janssen Pharmaceuticals, Takeda, Boehringer, Lundbeck. She has also participated in research funded by Janssen, Medtronic, Otsuka, Takeda.
Sanjay J. Mathew:
Over the past 3 years, Dr. Mathew has received consulting fees from Acadia, Alkermes, Allergan, Bracket, Cerecor, Fortress Biotech, Otsuka, and Valeant; He has received research support from: Janssen and NeuroRx. He has also received support from facilities and resources of the Michael E. Debakey VA Medical Center and the Johnson Chair for Research from Baylor College of Medicine.
Gerard Sanacora:
Dr. Sanacora has received consulting fees form Allergan, Alkermes, AstraZeneca, Biohaven Pharmaceuticals, Genentech, Janssen, Lundbeck, Merck, Navitor pharmaceuticals, Noven pharmaceuticals, Sage Pharmaceuticals, Takeda, Taisho Pharmaceuticals, Teva Pharmaceuticals and Vistagen Therapeutics over the last 36 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Johnson & Johnson, Hoffman La-Roche, Merck & Co., Naurex and Servier over the last 36 months. Free medication was provided to Dr. Sanacora for an NIH sponsored study by Sanofi-Aventis. In addition, he holds shares in Biohaven Pharmaceuticals and is a co-inventor on a patent “Glutamate agents in the treatment of mental disorders” Patent number: 8778979
Dan Iosifescu:
In the past three years, Dr. Iosifescu has received consultation fees from Alkermes, Axsome, MyndAnalytics (CNS Response), Jazz, Lundbeck, Otsuka, Sunovion, and has received research support (through his academic institutions) from Alkermes, Astra Zeneca, Brainsway, LiteCure, Neosync, Roche, Shire.
Charles DeBattista
Dr. DeBattista has received grant support from Janssen, Neuronetics, St. Jude, and Biolite. He has served on the Advisory Board of Alkermes.
Madhukar Trivedi:
Consulting/Advisory Board: Alkeremes Inc., Akili Interactive, Allergan Pharmaceuticals, Arcadia Pharmaceuticals, Avanir Pharmaceuticals, Brintellix Global, Bristol Myers Squibb, Caudex, Cerecor, Forest Pharmaceuticals, Global Medical Education Inc, Health Research Associates, Insys, Johnson & Johnson Pharmaceutical Research & Development, Lilly Research Laboratories, Lundbeck Research USA, Medscape, Merck & Co. Inc, Mitsubishi Pharma, MSI Methylation Sciences – Pamlab Inc., Navitor, Otsuka America Pharmaceutical Inc., One Carbon Therapeutics, Otsuka America Pharmaceutical Inc., Pfizer Inc, Takeda Global Research
Royalties: Janssen Research and Development LLC
Author Agreement: Janssen Asia Pacific, Oxford University Press
Honoraria: American Psychiatric Association
Grants: Agency for Healthcare Research and Quality (AHRQ), Cancer Prevention and Research Institute of Texas (CPRIT), National Institute of Mental Health (NIMH), National Institute of Drug Abuse (NIDA), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Center for Advancing Translational Sciences (NCATS), Johnson & Johnson, PCORI.
Maurizio Fava:
Reports 3-year disclosures as below -
All lifetime disclosures can be view on line at: http://mghcme.org/faculty/faculty-detail/maurizio_fava
Research Support:
Alkermes, Inc., Johnson & Johnson, Axsome, Acadia Pharmaceuticals, Cerecor, Lundbeck Inc., Neuralstem, Otsuka, Taisho, Marinus Pharmaceuticals, BioHaven, Takeda, Vistagen, Relmada Therapeutics Inc., Stanley Medical Research Institute (SMRI), National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH), and PCORI.
Dr. Fava has not done any personal consulting. Any consulting he has done has been on behalf of Massachusetts General Hospital.
Stock/Other Financial Options:
Equity Holdings: Compellis; PsyBrain, Inc.
Royalty/patent, other income: Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD) (US_7840419, US_7647235, US_7983936, US_8145504, US_8145505); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven. Patents for pharmacogenomics of Depression Treatment with Folate (US_9546401, US_9540691).
Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte.Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AP, A., 2000. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM-IV-TR®: American Psychiatric Association. [Google Scholar]
- Bech P, Allerup P, Gram LF, Reisby N, Rosenberg R, Jacobsen O, Nagy A, 1981. The Hamilton depression scale. Evaluation of objectivity using logistic models. Acta Psychiatr Scand 63(3), 290–299. [DOI] [PubMed] [Google Scholar]
- Bech P, Boyer P, Germain JM, Padmanabhan K, Haudiquet V, Pitrosky B, Tourian KA, 2010. HAM-D17 and HAM-D6 sensitivity to change in relation to desvenlafaxine dose and baseline depression severity in major depressive disorder. Pharmacopsychiatry 43(7), 271–276. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM, 1998. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11(1), 125–136. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M, 2013. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Celia Moreira Borella V, Seeman MV, Carneiro Cordeiro R, Vieira dos Santos J, Romario Matos de Souza M, Nunes de Sousa Fernandes E, Santos Monte A, Maria Mendes Vasconcelos S, Quinn JP, de Lucena DF, Carvalho AF, Macedo D, 2016. Gender and estrous cycle influences on behavioral and neurochemical alterations in adult rats neonatally administered ketamine. Dev Neurobiol 76(5), 519–532. [DOI] [PubMed] [Google Scholar]
- Chen WY, Huang MC, Lin SK, 2014. Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Laws KR, 2015. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 30(3), 152–163. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH, 2016. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22(3), 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman M, Flynn M, Judge H, Hoeppner B, Cusin C, Ionescu D, Mathew S, Chang L, Iosifescu D, Murrough J, DeBattista C, Schatzberg A, Trivedi M, Jha M, Sanacora G, Wilkinson S, Papakostas G, in press Double-Blind, Placebo-Controlled, Dose-Ranging Trial of Intravenous Ketamine as Adjunctive Therapy in Treatment-Resistant Depression (TRD). Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM, 2015. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290, 49–60. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Walker R, Laughren TP, Miller KK, Fava M, 2013. Female reproductive life cycle and hormones: methodology to improve clinical trials. J Clin Psychiatry 74(10), 1018–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, Sodhi MS, 2015. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 20(9), 1057–1068. [DOI] [PubMed] [Google Scholar]
- Guo R, Tang Q, Ye Y, Lu X, Chen F, Dai X, Yan Y, Liao L, 2016. Effects of gender on ketamine-induced conditioned placed preference and urine metabonomics. Regulatory toxicology and pharmacology : RTP 77, 263–274. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Wozniak DF, Benshoff ND, Olney JW, 2001. A comparative evaluation of the neurotoxic properties of ketamine and nitrous oxide. Brain Res 895(1-2), 264–267. [DOI] [PubMed] [Google Scholar]
- Kara NZ, Agam G, Anderson GW, Zitron N, Einat H, 2017. Lack of effect of chronic ketamine administration on depression-like behavior and frontal cortex autophagy in female and male ICR mice. Behav Brain Res 317, 576–580. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Pedersen RD, Holland PJ, Nemeroff CB, Rothschild AJ, Thase ME, Trivedi MH, Ninan PT, Keller MB, 2014. Influence of sex and menopausal status on response, remission, and recurrence in patients with recurrent major depressive disorder treated with venlafaxine extended release or fluoxetine: analysis of data from the PREVENT study. The Journal of clinical psychiatry 75(1), 62–68. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB, 2000. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 157(9), 1445–1452. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Toups M, Rush AJ, Wisniewski SR, Thase ME, Luther J, Warden D, Fava M, Trivedi MH, 2013. Do menopausal status and use of hormone therapy affect antidepressant treatment response? Findings from the Sequenced Treatment Alternatives to Relieve Depression (STAR* D) study. Journal of women’s health 22(2), 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Wohlreich MM, Mallinckrodt CH, Watkin JG, Stewart DE, 2006. Duloxetine efficacy for major depressive disorder in male vs. female patients: data from 7 randomized, double-blind, placebo-controlled trials. J Clin Psychiatry 67(5), 761–770. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR, 1986. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull 22(2), 343–381. [PubMed] [Google Scholar]
- Liebe T, Li S, Lord A, Colic L, Krause AL, Batra A, Kretzschmar MA, Sweeney-Reed CM, Behnisch G, Schott BH, Walter M, 2017. Factors Influencing the Cardiovascular Response to Subanesthetic Ketamine: A Randomized, Placebo-Controlled Trial. Int J Neuropsychopharmacol 20(11), 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston A, Waterman AE, 1977. Influence of age and sex on the duration of action ketamine in the rat [proceedings]. Br J Pharmacol 59(3), 491P. [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Guevara S, Machado-Vieira R, Richards EM, Brutsche NE, Nolan NM, Zarate CA Jr., 2014. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry 75(5), e417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan RL, Fava M, Agustin C, Baer L, Rosenbaum JF, 1997. Sensitivity of the six-item Hamilton Depression Rating Scale. Acta Psychiatr Scand 95(5), 379–384. [DOI] [PubMed] [Google Scholar]
- Posner K, Oquendo MA, Gould M, Stanley B, Davies M, 2007. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry 164(7), 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen KG, 2014. Psychiatric side effects of ketamine in hospitalized medical patients administered subanesthetic doses for pain control. Acta Neuropsychiatr 26(4), 230–233. [DOI] [PubMed] [Google Scholar]
- Salvadore G, van der Veen JW, Zhang Y, Marenco S, Machado-Vieira R, Baumann J, Ibrahim LA, Luckenbaugh DA, Shen J, Drevets WC, Zarate CA Jr., 2012. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol 15(8), 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, American Psychiatric Association Council of Research Task Force on Novel, B., Treatments, 2017. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry 74(4), 399–405. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M, 2016. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol Psychiatry 80(6), 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DM, Kornstein SG, 2003. Gender differences in depression and response to antidepressant treatment. Psychiatr Clin North Am 26(3), 581–594. [DOI] [PubMed] [Google Scholar]
- Tang WK, Morgan CJ, Lau GC, Liang HJ, Tang A, Ungvari GS, 2015. Psychiatric morbidity in ketamine users attending counselling and youth outreach services. Subst Abus 36(1), 67–74. [DOI] [PubMed] [Google Scholar]
- Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM, 2016. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res 312, 305–312. [DOI] [PubMed] [Google Scholar]
- Tundo A, de Filippis R, Proietti L, 2015. Pharmacologic approaches to treatment resistant depression: Evidences and personal experience. World J Psychiatry 5(3), 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Buuse M, Mingon RL, Gogos A, 2015. Chronic estrogen and progesterone treatment inhibits ketamine-induced disruption of prepulse inhibition in rats. Neurosci Lett 607, 72–76. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr., Sanacora G, 2018. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data MetaAnalysis. Am J Psychiatry 175(2), 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KN, Strong CE, Addonizio MN, Brownstein NC, Kabbaj M, 2017. Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle. Psychopharmacology (Berl) 234(3), 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, Balasubramani GK, Fava M, Trivedi MH, John Rush A, 2009. Sex differences in response to citalopram: a STAR*D report. Journal of psychiatric research 43(5), 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]


