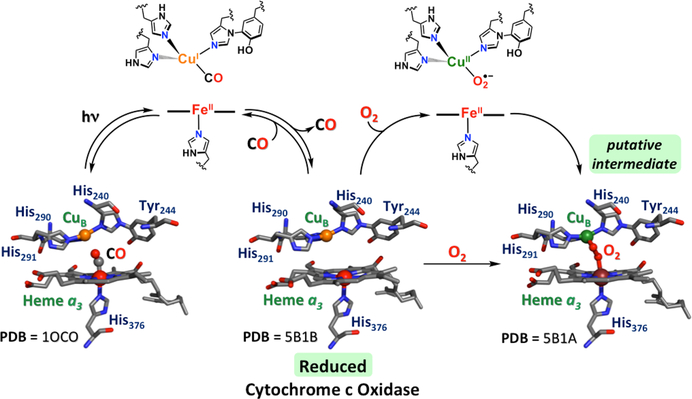
Figure 116.
Scheme depicting the dioxygen binding to bovine CcO for most kinetic-spectroscopic studies where a heme a3-CO adduct is utilized for experiments. Laser flash photolysis results in picosecond transfer to give a Cubi-CO species, whereupon CO either rebinds to the heme a3 or it escapes into solution. When the latter occurs, in the presence of dissolved O2(g), the fully reduced enzyme (PDB ID: 5B1B, center) can react with O2, giving a transient Cub-O2 adduct before it transfers to heme a3. In this scheme, that latter species is depicted as a peroxo-bridged Fea3III-CubII complex (PDB ID: 5B1A), many of which have been structurally characterized. It is generally not considered that this exact peroxide-bridged form (in its details) is an intermediate during enzyme turnover. Also, see the text.