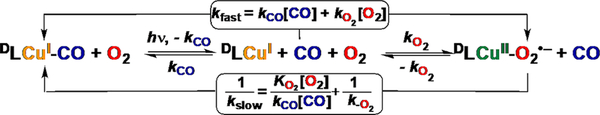
Figure 119.
Schematic employed to experimentally determine reaction kinetics [i.e., the 1:1 primary interaction of O2 and CO with various [(ligand)CuI] complexes (ligand = dL)], using transient absorption spectroscopy and the “flash-and trap” approach.940,1002 Experiments are conducted in the presence of precisely determined concentrations of O2 and CO (as mixtures) within the temperature range of interest and on nanosecond and longer time scales. As depicted here, initial laser photolysis of DLCuI-CO leads to CO photoejection, giving solvated dLCuI in a solution of both CO and O2. The initial “fast” process (kfast) involves the competitive binding of both CO and O2 with DLCuI, either regenerating DLCuI-CO or forming DLCuII-O2•−; thus, kfast is a combination of two rate constants, kO2 and kco. The latter is independently determined from transient absorption experiments with DLCuI–CO with only CO(g) present (i.e., in the absence of dioxygen). A complementary determination of the key parameters comes from observations that binding of carbon monoxide is thermodynamically favored compared to dioxygen, as is well-known for heme proteins and is also the case in copper-dioxygen proteins and complexes, Kco ≫ Ko2 (where K is the binding constant). Thus, CO subsequently displaces coordinated O2 from DLCuII-O2•− to reform the initial DLCuI–CO species (kslow; millisecond time scale), according to the equation for 1/kslow shown in the graphic, as originally deduced by Antonini and Burnori1255 in studies of heme protein O2-carriers. Adapted from ref 1002. Copyright 2010 American Chemical Society.