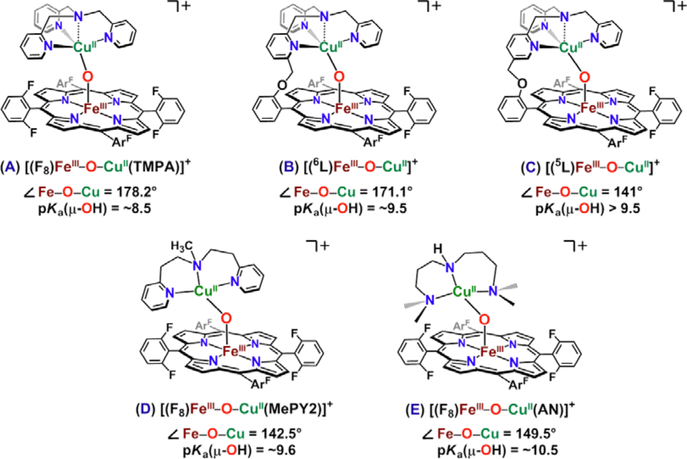
Figure 139.
Synthetic heme-copper μ-oxo complexes. The pKa values given are for H2O, estimated from those determined in MeCN by subtracting 7.5 pKa units. Except for complex C, all other μ-oxo complexes can be protonated with weak acids to give μ-hydroxo species. However, in all cases, the bridge breaks if stronger acids are added, even one equiv, to yield species such as [(5L)FeIIIOH (S)-CuII]+, where S = solvent or counteranion which is present. Thus, ligand environment can measurably influence the acid-base properties of μ-hydroxo/oxo heme-copper assemblies. See text and also section 6.3 for discussion of possible implications for HCO active site chemistry. See text for details.