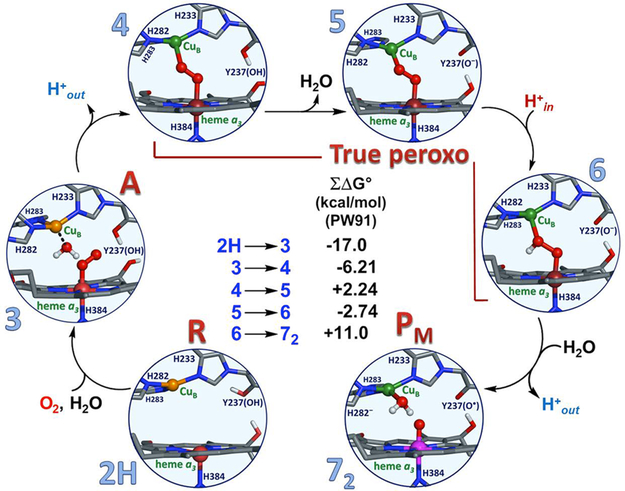
Figure 162.
Calculated structures and relative energies of proposed intermediates in the oxidative phase of the catalytic cycle of CcO, from Noodleman and co-workers.909 The pathway from 2H (reduced binuclear center intermediate R) to 6 contains low-energy barriers and is overall energetically downhill. The three so-called “true peroxo” states (4, 5, and 6) are all calculated to be lower in energy that state 3 (intermediate A). Further descriptors of the various calculated intermediates are 2H: {H376H+, FeII_CuI, H282H, Y'OH}; 3: {H376H+, FeII–O2_CuI–H2O, H282H, Y'OH}; 4: {H376, H2O FeIII–O–O–CuII, H282H, Y'OH}; 5: {H376H+, FeIII–O–O–CuII, H282H, Y'O‒}; 6: {H376H+, FeIII–O–OH–CuII, H282H, Y'O‒}; 72: {H376, H2O FeIV=O, H2O–CuII, H282‒, Y'O·•}. H376 is a nearby histidine residue with H-bonding interactions with copper ligand H283 through an aspartate residue and water molecule. H282 is one of the histidine residues bound to Cub. Y'O(H) is the Tyrosine residue cross-linked to His233; it may be deprotonated (i.e., Y'O‒) or have lost an H atom and be in its oxidized radical form (i.e., Y'O•).