Table 2.
Product Distribution of Cyclohexanecarboxyaldehyde Deformylation Reactivity Mediated by Various Heme Iron(III)-peroxo Adducts As Reported by Watanabe and Co-workersa
| percent yields of products [%]a |
|||
|---|---|---|---|
| Entry | Compound | 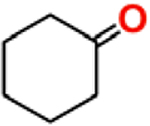 |
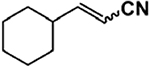 |
| 1 | [(TPP)FeIII-(O2)]− | 15 | 20 |
| 2 | [(TPP)FeIII-(O2)]−/PBNb | 8 | 15 |
| 3 | [(TMP)FeIII-(O2)]− | trace | 50 |
| 4 | [(TDCPP)FeIII-(O2)]− | 18 | 5 |
| 5 | KO2 | trace | 40 |
Adapted with permission from ref 551. Copyright 1998 Elsevier.
Yield were determined by GC/MS based on peroxoiron(III) prophyrin complex used.
[PBN] = 10 mM (PBN: phenyl-tert- butylnitrone).
