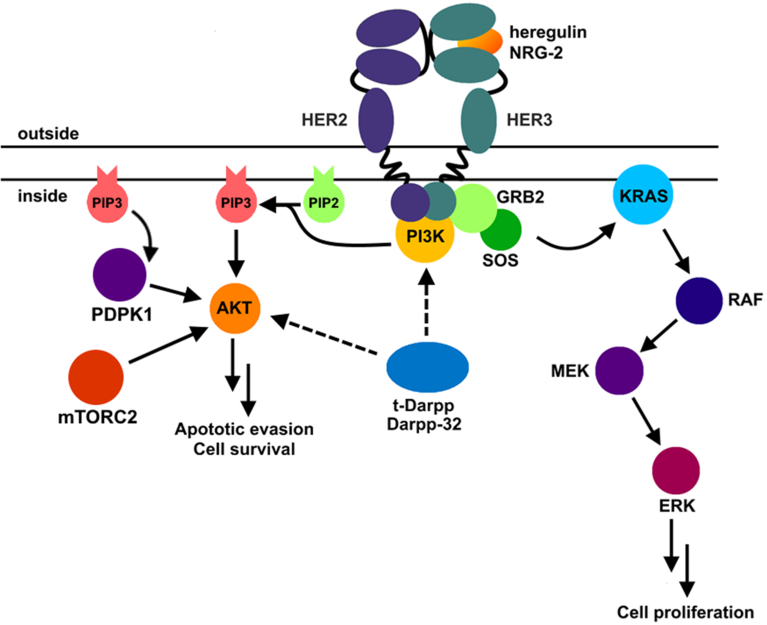
Figure 3. Her2 signaling pathway in breast cancers.
Her2 forms a heterodimer with Her3 (bound to its natural ligand, either heregulin or NRG-2). Productive dimerization causes transphosphorylation of cytoplasmic domains. PI3K binds the cytoplasmic domains and produces phosphatidylinositol (3,4,5)-trisphosphate (PIP3), a small molecule that activates 3-phosphoinositide-dependent protein kinase 1 (PDPK1). PDPK1 phosphorylates AKT at Serine 473, leading to apoptotic evasion and cell survival. t-Darpp and Darpp-32 activates AKT through a mechanism that is not yet clear, likely through PI3K signal strengthening or through another pathway that causes S473 phosphorylation on AKT. Another pathway activated by Her2 is the KRAS pathway. Here GRB2 adaptor protein activates SOS guanine nucleotide exchange protein to replace GDP with GTP on KRAS. KRAS instigates a cascade of phosphorylation reactions starting with RAF and ending in ERK, which binds to transcription factors and activates proliferation genes.