Fig. 3.
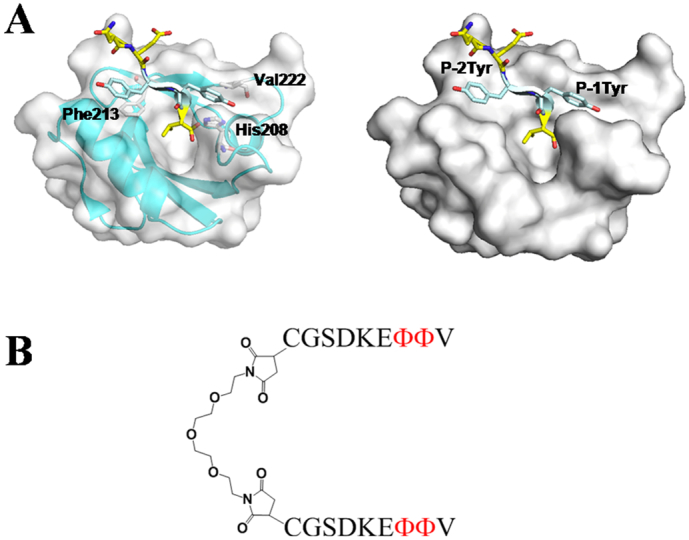
Syntenin and its peptide binding properties. (A) The structure of syntenin PDZ2 bound with the peptide NEYYV (PDB ID: 1w9o). Both tyrosine residues at P-1 and P-2 reside at hydrophobic clefts of the binding site, and interact with His 208, Val 222, and Phe 213, respectively [10]. (B) The structure of dimeric peptides based on peptide p3(DKEYYV). Substituted the tyrosine at P-1 and P-2 positions of peptide p3 (DKEYYV) by tryptophan, phenylalanine and an unnatural amino acid naphthylalanine (Φ) to increase the hydrophobicity at this position.
