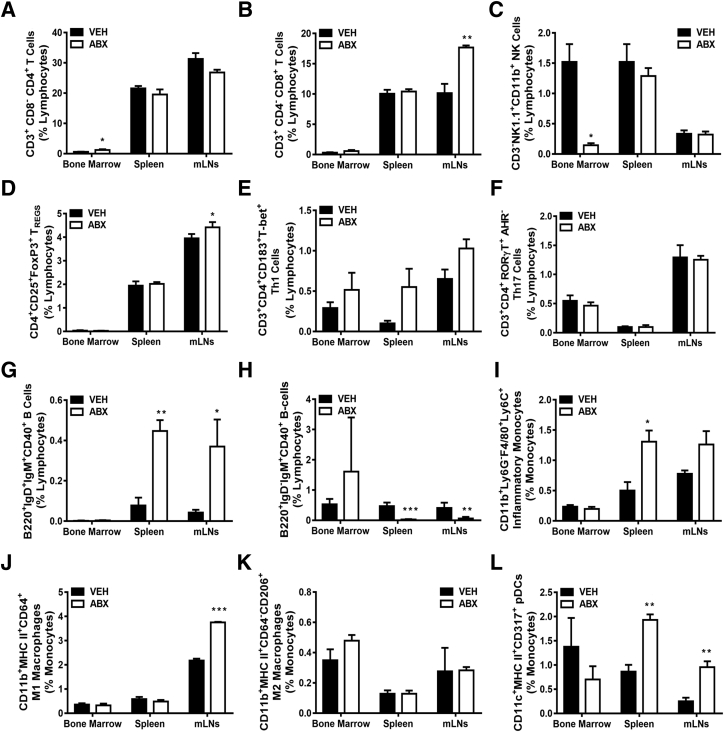
Figure 6.
Antibiotic (ABX) alteration of gut microbiota impact of innate and adaptive immune cell profile. Twelve-week–old male vehicle (VEH)– and ABX-treated mice were euthanized; specimens were harvested for analysis. A–H: Flow cytometric analysis of lymphocyte immune cell populations in bone marrow, spleen, and mesenteric lymph nodes (mLNs). Percentages of CD3+CD8−CD4+ (helper) T cells (A); CD3+CD4−CD8+ (cytotoxic) T cells (B); CD3−NK1.1+CD11b+ NK cells (C); CD3+CD4+CD25+FoxP3+ TREG cells (D); CD3+CD4+CD183+T-bet+ Th1 cells (E); CD3+CD4+RORγT+AHR− Th17 cells (F); B220+IgD+IgM+CD40+ (activated) B cells (G); and B220+IgD−IgM+CD40+ (memory) B cells (H). Percentages are expressed relative to total live gated lymphocytes. I–L: Flow cytometric analysis of innate immune cell populations in bone marrow, spleen, and mLNs. Percentages of CD11b+Ly6G−F4/80+Ly6C+ (proinflammatory) monocytes (I); CD11b+ MHC-class II+CD64+ M1 macrophages (J); CD11b+MHC II+CD64−CD206+ M2 macrophages (K); and CD11c+MHC-class II+CD317+ (activated plasmacytoid DCs; L). Cell percentages are expressed relative to total live-gated monocyte cells. Unpaired t-test was used. Data are expressed as means ± SEM (A–L). n = 4 to 5 per group (A–L). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus vehicle.