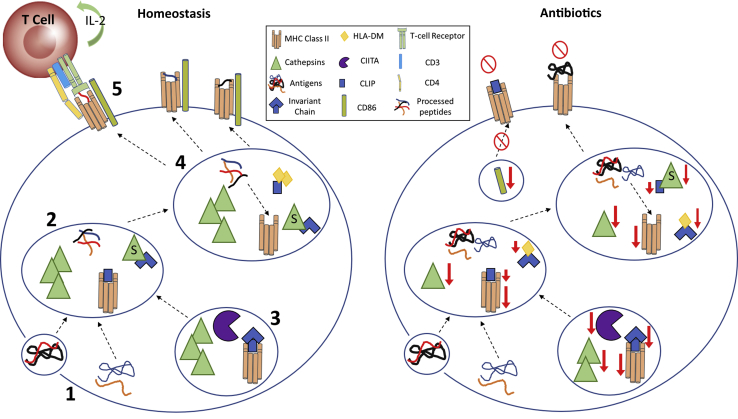
Figure 8.
Schematic of antibiotic effects on MHC class II Ag processing/presentation. 1: Under homeostatic conditions, Ags of exogenous/endogenous nature are taken up by APCs and processed by cathepsins within the endosomal/lysosomal compartments. 2: Cathepsins B, C, and D process Ags by unfolding and splicing these Ags into smaller, functionally active peptides. 3: Simultaneously, the MHC class II transactivator (CIITA) regulates MHC class II α/β heterodimer transcription, leading to class II components being synthesized within the endoplasmic reticulum to form a complex with invariant chain (Ii). This complex is further processed in the endosomal/lysosomal compartments by cathepsins S and L to degrade Ii, leaving class II–associated Ii peptide (CLIP) in the class II binding groove to block low-affinity peptides from prematurely binding to class II complexes. 4: The nonclassic class II molecule, human leukocyte antigen (HLA)-DM, chaperones the removal of CLIP from the peptide binding groove and catalyzes the loading of high-affinity peptides, leading to a stable MHC class II–peptide complex. 5: This complex is then transported to the cell surface for CD4+ T-cell recognition, with costimulation by proteins such as CD80/CD86. However, when antibiotics alter the gut microbiota, dysregulation is detected in several genes responsible for proper MHC class II–Ag processing and presentation. Without proper Ag processing and presentation, T cells may not develop tolerance to self-Ag, and APCs are less likely to clear long-term immune challenges.