Figure 3.
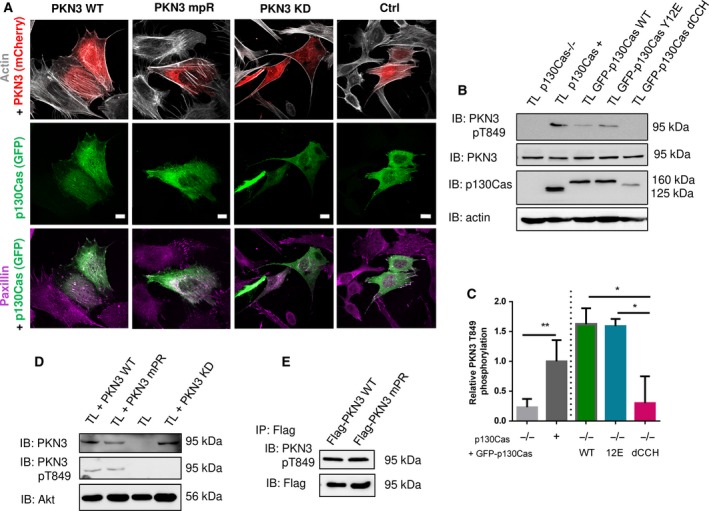
PKN3 activity is important for stress fibers formation and is stimulated by the expression of p130Cas. (A) p130Cas−/− MEFs growing on FN‐coated cover slips were co‐transfected by GFP‐p130Cas and mCherry‐PKN3 fusion variant (WT, mPR, KD) or mCherry. After 48 h, cells were fixed and imaged by Leica TCS SP2 microscope (63×/1.45 oil objective). Stress fibers were visualized by Phalloidin (405) and focal adhesions by anti‐Paxillin staining (2nd 633). Representative images are shown. Scale bars represent 20 μm. (B) p130Cas−/− MEFs or p130Cas−/− MEFs re‐expressing p130Cas or transfected by GFP‐fused p130Cas variants (WT, YE, dCCH) were lysed in RIPA buffer, blotted to nitrocellulose membrane, and analyzed for endogenous PKN3 activity by antibody anti‐phosphoThr849 of PKN3 (pT849 PKN3). Expression of p130Cas mutants was verified by anti‐p130Cas antibody and loading by anti‐PKN3 and anti‐actin antibody. (C) Densitometric quantification of PKN3 activity (pT849 PKN3 phosphorylation). The effect of p130Cas re‐expression on PKN3 T849 phosphorylation was analyzed separately from the effect of transfected p130Cas mutants (indicated by a dotted line). Error bars indicate means ± SD from three independent experiments (four experiments for the left part). Statistical significance was evaluated by one‐way repeated ANOVA followed by Turkey's post hoc test (*P < 0.05; **P < 0.01). (D) Lysates or (E) immunoprecipitates (by Flag sepharose) from p130Cas−/− MEFs re‐expressing p130Cas and overexpressing PKN3 variants (WT, mPR, KD) were immunoblotted by anti‐PKN3, anti‐pT849 PKN3, and anti‐Akt antibodies (loading control).
