Abstract
Objectives:
Despite the successes of treatment with antiretroviral therapy in reducing morbidity and mortality among HIV-infected patients, long-term sustainability of the initial regimen has become challenging. Therefore, this study is aimed to address pattern of and reasons for change of antiretroviral therapy regimens among HIV/AIDS patients at Jugel Hospital, Eastern Ethiopia.
Methods:
A retrospective cross-sectional study was conducted to review medical records of 220 patients who had been on treatment and experienced regimen change at least once from September 2006 to August 2016. Structured data abstraction format was customized from World Health Organization guideline. Data were entered in Epi-data version 3.1, and exported to and analyzed with Statistical Package for Social Sciences version 20. Following descriptive statistics, binary logistic regression was run to determine the association between selected variables and second-time regimen change.
Results:
The mean age of patients was 37.6 (±8.9) years and 62.3% of them were female. Majority of the patients were presented to the hospital with World Health Organization clinical stage III (59.1%) and CD4 count below 200 cells/mm3 (68.6%). The mean duration of stay on initial regimen was found to be 3.26 (±1.92) years. The average number of initial regimen changes per year was 22 (±11.28). In two-thirds (66.36%) of the patients, their initial regimen was changed to tenofovir disproxil fumarate–based alternatives. The most-frequent reason for initial regimen change was toxicity (32.3%). Among those who experienced the regimen change for the first time, the prevalence of second-time regimen change was found to be 18.18%. Patients who had been taking tuberculosis treatment along with antiretroviral therapy were more likely to get their regimen changed for the second-time compared to those who were not infected with tuberculosis (adjusted odds ratio: 3.40; 95% confidence interval: 1.87–6.47). Besides, patients who were on zidovudine-based (adjusted odds ratio: 0.26; 95% confidence interval: 0.33–0.47) and tenofovir disoproxil fumarate–based regimens (adjusted odds ratio: 0.03; 95% confidence interval: 0.01–0.12) were less likely to get their regimen changed for the second-time compared to those who were on stavudine-based regimens.
Conclusion:
The majority of the patients had their treatment regimen changed because of drug-related toxicities, treatment failure, and comorbid conditions. Some regimen changes might be attributable to failure of either hospital supply system or patient-related factors which would have been prevented considering limited number of treatment options. There must be consideration of risks and benefits prior to changing a particular regimen.
Keywords: Regimen change, antiretroviral therapy, HIV/AIDS, adults, Ethiopia
Introduction
Since 2005, the number of new HIV/AIDS cases each year has dropped 22%, the global HIV/AIDS death rate declined 52%, and 20.9 million people are now on ART.1 These historic gains were made, in part, because of the extraordinary amount of resources made available to fight HIV/AIDS. Despite successes of treatment with ART in reducing morbidity and mortality among HIV-infected patients, long-term sustainability of the initial therapeutic regimen has become challenging.2 The HIV-infected people once start the treatment, they are supposed to remain on the treatment throughout their life-time.3 However, at least one of the medications in the initial ART regimen has been changed due to various reasons involving toxicities, treatment failure, and poor adherence or simplification, among others.4–6 The pattern of the ART regimen change also varies over time.6 For instance, estimated probabilities of changing at least one medication during periods of therapy cumulatively increased with duration of stay on the therapy.7 However, early medication change within 2 months of ART initiation was reported in many patients,8 and the primary reason for the initial regimen change was toxicity or intolerance.2,7–9 Similarly, ART regimen change during the first year of initial therapy is mostly due to toxicities.10 Among the regimens initiated, stavudine (d4T)/lamivudine (3TC)/nevirapine (NVP) is the regimen with the foremost experience of change.3,10–12
As per the World Health Organization (WHO) guideline, first-line ART regimen should mainly consist of two nucleoside reverse-transcriptase inhibitors (NRTIs) plus a non-nucleoside reverse-transcriptase inhibitor (NNRTI). From which, tenofovir disoproxil fumarate (TDF) with 3TC or emtricitabine (FTC) and efavirenz (EFV) as a fixed-dose combination is recommended as the preferred option to initiate ART. In the year 2010, WHO updated a guideline emphasizing the withdrawal of d4T use from first-line regimens because of its well-recognized metabolic toxicities.13–15
ART, as a healthcare program, is run by various stakeholders including governmental and non-governmental organizations. Despite collaboration of stakeholders for effectiveness of the program, it is facing new challenges from different perspectives, and regimen change is the key among the challenges. Although moving away from medications with poor safety profiles (e.g. d4T) could improve regimen tolerability,2,5 a regimen change from preferred first-line to second-line of therapy could narrow the opportunities of treatment for optimal patient outcomes. Protease inhibitor-based ART regimens, for instance, are second-line regimens in adults and were found to be associated with increased rates of discontinuation and treatment failure.16 Therefore, it seems logical to evaluate reasons behind regimen change in order to preserve treatment options for the patients.6 Operationally, changes in policy or guideline and hence in medication(s) of a regimen could be made as a solution to improve patient outcomes. Hence, this study is aimed to assess the patterns of and reasons for ART regimen change among adult HIV/AIDS patients on treatment follow-up at Jugel Hospital (JH) HIV clinic.
Methods
Study setting, design, and period
This study was carried out in JH’s HIV clinic in Harar which is located 526 km away from the capital of Ethiopia, Addis Ababa to the East. JH has three main wards including medical, surgical and gynecology, and obstetrics wards. Besides, it provides service for medical, gynecology, eye, dental, and pediatric outpatient departments. It also has HIV and tuberculosis (TB) clinics. The hospital has also a suitable record-keeping system for HIV/AIDS patients following their treatments. Hospital-based cross-sectional study was employed to determine pattern of and reasons for ART regimen change among adult HIV/AIDS patients. The study was conducted from 1 June to 15 July 2017.
Population and inclusion/exclusion criteria
Adult HIV/AIDS patients (aged 15 years and above) who had been on the ART follow-up and who had experienced at least one regimen change at the HIV clinic of the hospital from September 2006 to August 2016 were considered as the study population. In this study, the d4T-based ART regimen was included to consider changes other than d4T within the regimen. In the d4T-based regimens (e.g. d4T/3TC/NVP and d4T/3TC/EFV), the change due to other drugs (NVP or EFV) were considered. However, exclusive change of d4T in the regimen (e.g. d4T/3TC/NVP → AZT/3TC/NVP) was not considered because of the official withdrawal of it by WHO with obvious reasons in 2010.14,15 Majority of the changes related to d4T was found unregistered due to such interventions.
Sample size determination and sampling techniques
To increase the representativeness and power of the sample size, it is worth-mentioning that all eligible medical records of HIV/AIDS patients served at the HIV clinic of the hospital from September 2006 to August 2016 were included. Therefore, 220 medical records were taken as a final sample size and no sampling technique was employed as all eligible records were considered based on the inclusion and exclusion criteria.
Variables and measurements
The study included only patients whose regimen had been changed for at least once. The outcome (response) variable was considered as regimen change. Socio-demographic characteristics (age, sex, marital status, and educational status), and clinical- and treatment-related characteristics (TB treatment, CD4 count, and types of ART regimen) of HIV/AIDS patients were considered as a predictor (independent) variables.
Data collection tool and process
Every relevant datum was abstracted from the medical records of HIV/AIDS patients whose regimen had been changed ART within the window of the review period. Thereafter, it was recorded into data collection checklist designed and customized from the WHO guideline.14 The data abstraction format included socio-demographic characteristics, clinical and immunological characteristics, and patterns of and reasons for first- and second-time regimen change. The checklist was prepared in such a way that the second-time regimen change was recorded with the same procedure followed for the initial regimen change. Two healthcare professionals (one pharmacist and one Nurse) working in the hospital were recruited and trained about the purpose and methods of data collection prior to the actual study.
Data quality control
Pretest was conducted in Hiwot Fana Specialized University Hospital, found in the same town, by taking 25 medical records (11.36%) of HIV/AIDS patients who had experienced regimen change at least once to ensure feasibility of the data collection format in resource limited settings. Necessary amendment was made accordingly on the format considering temporal and spatial differences. Data cleaning was also performed on daily basis. In addition, focused training was given for data collectors for better data acquisition.
Data processing and analysis
Data were entered in to Epi-data version 3.1 and exported to and analyzed with Statistical Package for Social Sciences (SPSS) version 20 (IBM Statistics, Armonk, NY, USA). Descriptive statistics (frequency and percentage distribution) was utilized to summarize socio-demographic, anthropometric, and clinical characteristics, as well as patterns of and reasons for ART regimen changes. Binary logistic regression analysis was performed to identify the association of some categorical variables with second-time regimen change. The covariate with p-value less than 0.25 by bivariate analysis was retained for the subsequent multivariate analysis. Significant association was declared at p-values less than 0.05 with 95% confidence level.
Results
Socio-demographic characteristics
In total, 220 eligible medical records of ART were reviewed to evaluate regimen change within the 10 years period. As summarized in Table 1, majority (n = 121, 55%) of the patients were between the ages of 30–45 years (mean = 37.6, standard deviation (SD) = 8.9). Besides, large proportion of patients who experienced change of regimen for at least once were female (n = 137, 62.3%), married (n = 109, 49.5%), and attended the primary education (n = 95, 43.2%) (Table 1).
Table 1.
Socio-demographic, anthropometric, and clinical characteristics of HIV/AIDS patients who changed their initial ART regimen at Jugel Hospital from September 2006 to August 2016.
| Characteristics | Frequency (%) | |
|---|---|---|
| Age (years), mean = 37.6 (±8.9) | 15–30 | 18 (8.2) |
| 30–45 | 121 (55.0) | |
| 45–60 | 64 (29.1) | |
| >60 | 17 (7.7) | |
| Sex | Male | 83 (37.7) |
| Female | 137 (62.3) | |
| Marital status | Single | 35 (15.9) |
| Married | 109 (49.5) | |
| Divorced | 48 (21.8) | |
| Widowed | 28 (12.7) | |
| Educational status | No formal education | 41 (18.6) |
| Primary | 95 (43.2) | |
| Secondary | 70 (31.8) | |
| Higher education | 14 (6.4) | |
| Weight on ART initiation (kg) | <45 | 48 (21.8) |
| 45–55 | 95 (43.2) | |
| >55 | 77 (35.0) | |
| WHO clinical staging | I | 26 (11.8) |
| II | 22 (10.0) | |
| III | 130 (59.1) | |
| IV | 42 (19.1) | |
| Baseline CD4 count (cell/mm3) | <200 | 151 (68.6) |
| 200–350 | 47 (21.4) | |
| 350–500 | 14 (6.4) | |
| >500 | 8 (3.6) | |
| Latest CD4 count (cell/mm3) | <200 | 21 (9.5) |
| 200–350 | 35 (15.9) | |
| 350–500 | 52 (23.6) | |
| >500 | 112 (50.9) | |
| Latest viral load (copies/dL) | ND | 182 (82.7) |
| 50–1000 | 2 (0.9) | |
| 1000–10,000 | 4 (1.8) | |
| >10,000 | 5 (2.3) | |
| Not examined | 27 (12.3) | |
| TB during initial change | Yes | 52 (23.64) |
| No | 168 (76.36) | |
| OI prophylaxis during initial regimen change (n = 220) | No prophylaxis | 7 (3.2) |
| INH | 8 (3.6) | |
| Cotrimoxazole | 92 (41.8) | |
| Cotrimoxazole and INH | 113 (51.4) | |
| OI prophylaxis during second regimen change (n = 40) | No prophylaxis | 5 (12.5) |
| INH | 7 (17.5) | |
| Cotrimoxazole | 7 (17.5) | |
| Cotrimoxazole and INH | 21 (52.5) | |
ART: antiretroviral therapy; WHO: World Health Organization; OI: opportunistic infections; ND: not detectable; INH: isoniazid; TB: tuberculosis.
Clinical characteristics
The majority (n = 130, 59.1%) of patients were at WHO clinical stage III during initiation of ART. The baseline CD4 count was less than 200 cells/mm3 for most of the patients (n = 151, 68.6%); however, in larger proportion of patients, the latest CD4 count and latest viral load were found to be greater than 500 cells/mm3 (n = 112, 50.9%) and undetectable (n = 182, 82.7%), respectively. Moreover, TB treatment was observed in some patients during the regimen change in the review period. Larger proportion of patients were taking cotrimoxazole and isoniazid prophylactic therapy concurrently in the first- (n = 113, 51.4%) and second-time regimen change (n = 21, 52.5%) (Table 1).
Distribution patterns of initial ART regimens
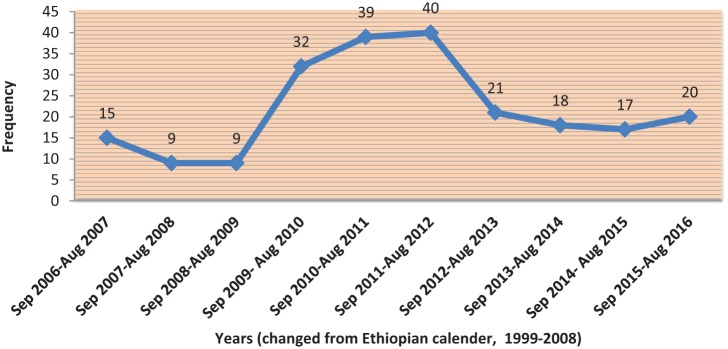
There was lower level of regimen change early (2006 to August 2009) and gradually increased around the mid of the review period (September 2009 to August 2012). However, in the late review period, the level of regimen change started to decline and remain stable thereafter. The average number of patients whose regimen had changed per year was found to be 22 with SD of ±11.28 (Figure 1). Besides, majority (37.7%) of the patients took their initial ART regimen for 2–4 years (mean, 3.26 ± 1.92) followed by those who took for less than 2 years (32.7%) (Figure 2). Concerning the initial ART regimens, almost three-fifths (n = 135, 61.35%) of the patients were initiated with d4T-based regimens. The d4T-based regimen mainly contained NNRTIs of either NVP (d4T/3TC/NVP) or EFV (d4T/3TC/EFV). The vast majority of patients were taking d4T/3TC/NVP regimen (n = 118, 53.63%), whereas small number of patients was on TDF-based regimens during initiation (Table 2).
Figure 1.
Distribution of initial regimen change from September 2006 to August 2016.
Figure 2.
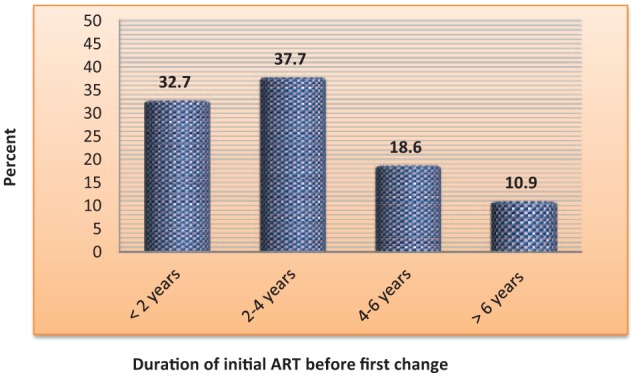
Duration of patients on initial ART regimen in years among HIV/AIDS patients in JH from September 2006 to August 2016.
Table 2.
Distribution of first-time regimen change by individual category at JH from September 2006 to August 2016.
| Initial ART regimen | Initial regimen changed to (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d4T-based |
AZT-based |
TDF-based |
ABC-based |
Total | ||||||
| d4T/3TC/NVP | d4T/3TC/EFV | AZT/3TC/NVP | AZT/3TC/EFV | TDF/3TC/EFV | TDF/3TC/NVP | TDF/3TC/LPV/r | TDF/3TC/ATZ/r | ABC/3TC/LPV/r | ||
| d4T/3TC/NVP | – | 25 (21.19) | a | 20 (16.95) | 67 (56.78) | a | 3 (2.54) | 1 (0.85) | 2 (1.69) | 118 (53.63) |
| d4T/3TC/EFV | 3 (17.65) | – | 3 (17.65) | a | a | 6 (35.29) | 4 (23.53) | 0 | 1 (5.88) | 17 (7.72) |
| AZT/3TC/NVP | 0 | 0 | – | 11 (32.35) | 13 (38.24) | 7 (20.59) | 3 (8.82) | 0 | 0 | 34 (15.45) |
| AZT/3TC/EFV | 1 (3.57) | 0 | 3 (10.71) | – | 20 (71.43) | 1 (3.57) | 1 (3.57) | 1 (3.57) | 1 (3.57) | 28 (12.72) |
| TDF/3TC/EFV | 0 | 0 | 0 | 0 | – | 4 (57.14) | 1 (14.28) | 1 (14.28) | 1 (14.28) | 7 (3.18) |
| TDF/3TC/NVP | 0 | 1 (6.25) | 0 | 0 | 11 (68.75) | – | 2 (12.5) | 0 | 2 (12.5) | 16 (7.27) |
| Total | 4 (1.82) | 26 (11.82) | 6 (2.73) | 31 (14.09) | 111 (50.45) | 18 (8.82) | 14 (6.36) | 3 (1.36) | 7 (3.18) | 220 |
TDF: tenofovir disoproxil fumarate; d4T: stavudine; AZT: zidovudine; ABC: abacavir.
Mere change of d4T was omitted.
Patterns of ART regimen changes
Generally, two-thirds of patients (n = 146, 66.36%) had their initial regimen changed to TDF-based regimens. Two of the regimens were first-line (TDF/3TC/EFV, 50.45% and TDF/3TC/NVP, 8.82%) and the remaining were second-line TDF-based alternatives (TDF/3TC/LPV/r, 6.36% and TDF/3TC/ATZ/r, 1.36%). Small number of patients (n = 7, 3.18%) had their initial regimen changed to second-line ABC-based regimen directly. During the review period, a change from other regimens (zidovudine (AZT)- or TDF-based) to d4T-based was considered. Similarly, the interchangeable use of either NNRTIs (NVP and EFV) in d4T-based regimen was considered. Before 2010, about 30 patients (13.63%) had their initial regimen changed to d4T-based regimens (Table 2). Among those who experienced initial regimen change, 40 (18.18%) of patients had their initial regimen changed regimen for the second-time. Observing the distribution of the regimen change, almost half (n = 19, 47.5%) of patients had their initial regimen changed to TDF-based regimens (TDF/3TC/EFV, 35%; TDF/3TC/NVP, 2.5%; and TDF/3TC/LPV/r, 10.0%). The remaining patients had their initial regimen changed to AZT-based first-line (n = 12, 30.0%) and ABC-based second-line regimens (n = 9, 22.5%) (Table 3).
Table 3.
Distribution of second-time regimen change at JH from September 2006 to August 2016.
| Regimen before second-time change | Second-time regimen changed to |
|||||||
|---|---|---|---|---|---|---|---|---|
| AZT-based |
TDF-based |
ABC-based |
Total | |||||
| AZT/3TC/NVP | AZT/3TC/EFV | TDF/3TC/EFV | TDF/3TC/NVP | TDF/3TC/LPV/r | ABC/3TC/LPV/r | ABC/3TC/ATZ/r | ||
| d4T/3TC/NVP | a | 0 | 1 (50.00) | a | 0 | 1 (50.00) | 0 | 2 |
| d4T/3TC/EFV | 4 (22.22) | a | a | 9 (50.00) | 5 (27.77) | 0 | 0 | 18 |
| AZT/3TC/NVP | – | 1 (25.00) | 2 (50.00) | 1 (25.00) | 0 | 0 | 0 | 4 |
| AZT/3TC/EFV | 1 (25.00) | – | 3 (75.00) | 0 | 0 | 0 | 0 | 4 |
| TDF/3TC/EFV | 0 | 0 | – | 0 | 4 (57.14) | 3 (42.86) | 0 | 7 |
| TDF/3TC/LPV/r | 0 | 0 | 0 | 0 | – | 0 | 1 (100.00) | 1 |
| ABC/3TC/LPV/r | 0 | 0 | 0 | 0 | 0 | – | 4 (100.00) | 4 |
| Total | 5 (12.5) | 7 (17.5) | 14 (35.0) | 1 (2.5) | 4 (10.0) | 4 (10.0) | 5 (12.5) | 40 |
TDF: tenofovir disoproxil fumarate; AZT: zidovudine; ABC: abacavir.
Changes due to stavudine alone should not be counted (it was already changed by WHO irrespective of specific reasons).
Reasons for regimen changes
Almost one-third of patients (n = 71, 32.3%) with first-time regimen change had a record of toxicities as a reason for regimen change. Among the toxicities documented; skin rash (n = 40, 56.33%), anemia (n = 14, 19.72%) and central nervous system (CNS) toxicities (n = 11, 15.49) were the common toxic effects (Table 4). Anemia was reported from AZT-based regimen, whereas skin rash and CNS toxicities were reported from NVP- and EFV-containing regimens, respectively (Table 5). The second and third most common reason for first-time regimen change were comorbidity (n = 52, 23.64%) and treatment failure (n = 34, 15.5%), respectively. Observing the treatment failure, near to two-thirds (64.70%) of them experienced immunologic failure (drop in CD4 count). Some patients experienced immunologic, virologic, and clinical failures concurrently (11.76%) (Table 5). The primary documented reasons for the second-time regimen change were drug-related toxicities (32.5%) and treatment failures (27.5%) (Figure 3).
Table 4.
Documented reasons for first-time change among HIV/AIDS patients at JH from September 2006 to August 2016.
| Documented reasons for first-time regimen change | Frequency (%) |
|---|---|
| Toxicity | 71 (32.3) |
| Rash | 40 (56.33) |
| Anemia | 14 (19.72) |
| CNS toxicities | 11 (15.49) |
| Hepatotoxicity | 4 (5.63) |
| Lipodystrophy | 1 (1.41) |
| Jaundice | 1 (1.41) |
| Treatment failure | 34 (15.5) |
| Immunologic | 22 (64.70) |
| Clinical | 2 (5.88) |
| Virologic | 1 (2.94) |
| Immunologic and clinical concurrently | 5 (14.71) |
| Immunologic, virologic, and clinical concurrently | 4 (11.76) |
| Comorbidity | 52 (23.64) |
| Adherence difficulty | 29 (13.18) |
| Pregnancy | 7 (3.18) |
| Stock-out | 5 (2.27) |
| Othersa | 22 (10.0) |
| Total | 220 (100) |
Others include patient inconvenience, lost to follow-up, undocumented reasons.
Table 5.
Toxicity profile as a reason for change.
| Initial ART regimen | Toxicity profile as a reason for change |
Subtotal | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anemia | CNS toxicities | Lipodystrophy | Rash | Jaundice | Hepatotoxicity | ||||
| d4T-based | d4T/3TC/NVP | a | – | a | 30 (42.25) | 1 (1.41) | 4 (5.63) | 35 | 41 |
| d4T/3TC/EFV | a | 6 (8.45) | a | 0 | 0 | 0 | 6 | ||
| AZT-based | AZT/3TC/NVP | 8 (11.27) | 0 | 1 (1.41) | 7 (9.86) | 0 | 0 | 16 | 26 |
| AZT/3TC/EFV | 6 (8.45) | 4 (5.63) | 0 | 0 | 0 | 0 | 10 | ||
| TDF-based | TDF/3TC/EFV | 0 | 1 (1.41) | 0 | 0 | 0 | 0 | 1 | 4 |
| TDF/3TC/NVP | 0 | 0 | 0 | 3 (4.22) | 0 | 0 | 3 | ||
| Total | 14 (19.71) | 11 (15.49) | 1 (1.41) | 40 (56.33) | 1 (1.41) | 4 (5.63) | 71 (100) | ||
TDF: tenofovir disoproxil fumarate.
d4T-related toxicities were omitted due to intervention of WHO (banned from market due to obvious reasons).
Figure 3.
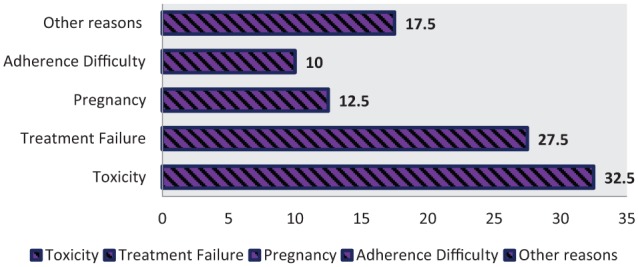
Reason for second-time regimen change.
Factors associated with second-time regimen changes
Those patients who had been taking TB treatment along with ART were more likely to experience change of regimen for the second-time compared to those who did not have TB (adjusted odds ratio (AOR): 3.40; 95% confidence interval (CI): 1.87–6.47). Besides, the type of ART regimens showed significant but negative association with second-time regimen change. Those patients who were on AZT-based (AOR: 0.26; 95% CI: 0.33–0.47) and TDF-based regimens (AOR: 0.03; 95% CI: 0.01–0.12) were less likely to get their regimen changed for the second-time compared to d4T-based regimens (Table 6).
Table 6.
Binary logistic regression analysis showing the association between socio-demographic and clinical factors with second-time regimen change.
| Variables | Regimen changed for second time |
COR | p-value | AOR | ||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Age (years) | 14–30 | 2 | 16 | 1 | – | 1 |
| 30–45 | 20 | 101 | 1.58 (0.34–7.43) | 0.56 | 1.73 (0.21–14.3) | |
| 45–60 | 17 | 47 | 2.89 (0.60–13.92) | 0.19 | 2.96 (0.34–25.7) | |
| >60 | 1 | 16 | 0.50 (0.041–6.08) | 0.59 | 1.67 (0.08–33.97) | |
| Sex | Male | 17 | 66 | 1 | – | |
| Female | 23 | 114 | 0.78 (0.39–1.57) | 0.49 | ||
| Marital status | Single | 10 | 25 | 1 | – | 1 |
| Married | 21 | 88 | 0.59 (0.25–1.43) | 0.25 | 0.37 (0.11–1.26) | |
| Divorced | 6 | 42 | 0.36 (0.116–1.10) | 0.07 | 0.45 (0.09–2.16) | |
| Widowed | 3 | 25 | 0.30 (0.074–1.22) | 0.09 | 0.17 (0.03–1.08) | |
| Educational status | No formal education | 4 | 37 | 1 | – | 1 |
| Primary | 17 | 78 | 2.01 (0.63–6.41) | 0.23 | 3.09 (0.60–15.84) | |
| Secondary | 16 | 54 | 2.74 (0.84–8.85) | 0.09 | 3.66 (0.72–18.57) | |
| Higher education | 3 | 11 | 2.52 (0.48–13.0) | 0.29 | 8.79 (0.97–72.16) | |
| TB treatment | No | 19 | 149 | 1 | – | 1 |
| Yes | 21 | 31 | 5.3 (2.56–11.03) | 0.00 | 3.40 (1.87–6.47)* | |
| Latest CD4 count | <200 | 7 | 14 | 1 | – | 1 |
| 200–350 | 5 | 30 | 0.33 (0.09–1.23) | 0.10 | 0.36 (0.06–2.40) | |
| 350–500 | 9 | 43 | 0.42 (0.13–1.33) | 0.14 | 0.83 (0.14–4.82) | |
| >500 | 19 | 93 | 0.41 (0.14–1.15) | 0.089 | 0.61 (0.12–3.09) | |
| Regimen before second change | d4T-based | 20 | 10 | 1 | – | 1 |
| AZT-based | 8 | 31 | 0.13 (0.04–0.38) | 0.00 | 0.13 (0.33–0.47)* | |
| TDF-based | 8 | 36 | 0.03 (0.01–0.08) | 0.00 | 0.03 (0.01–0.12)* | |
| ABC-based | 4 | 3 | 0.67 (0.12–3.57) | 0.64 | 0.88 (0.12–6.41) | |
COR: crude odds ratio; AOR: adjusted odds ratio.
p-value less than 0.05.
Discussion
The HIV-infected people once initiated the treatment, they were supposed to remain on the treatment during their lifetime. However, at least one of the medications in the initial ART regimen has been changed due to various reasons.1,3,17 Early change in regimens contributes for narrowing the available treatment options to sustain nearly normal life. Therefore, this study is aimed to assess the patterns of and reasons for ART regimen change among adult HIV/AIDS patients on treatment follow-up at JH HIV clinic.
In this study, the majority of patients were within age range of 30–45 years with mean = 37.6 (±8.9) years (55%), female (62.3%), married (49.5%), and attended primary school (43.2%). In line with the present finding, several studies indicated that among patients who had their treatment changed for the first time, the majority was female.3,4,11,18–20 This might be due to the fact that the higher proportion of female patients who initially started ART and the increased likelihood of regimen change among female for specific reasons such as pregnancy. Looking at the age distribution of patients included in various studies, the mean ages of the participants were 40.2 (±8.0) years in major referral hospital of Southwest Cameroon,11 38.6 (±7.0) years and 36.5 (±8.9) years in health facilities of Addis Ababa, Ethiopia.3,18 Similarly, the age ranges of majority of patients were 20–34 years in Oromia Regional State, Ethiopia;19 31–45 years in Tikur Anbesa Specialized Hospital, Ethiopia;18 and 30–44 years in regional hospital of KwaZulu-natal, South Africa.20 With the exception of Wube et al. who reported younger adults, the majority of studies indicated that the large proportion of adults to whom their initial regimen changed were within late 30s to mid 40s. In agreement with our study, Niguse also reported that nearly half (46.3%) of the patients had got married.18 Even though, the scale-up of ART is expected to raise adult life expectancy in populations with high HIV prevalence, early change around the aforementioned age ranges might compromise the possibility of living nearly normal life span21
Regarding the anthropometric and clinical characteristics, larger proportion of patients weighed 45–55 kg (51 ± 9.3), presented with WHO clinical stage III, and CD4 count less than 200 cells/mm3 at initiation of ART regimen. However, majority of patients showed immunologic and virologic improvements as indicated by CD4 count and viral load measurement. Meanwhile at the initial regimen change, almost half of the patients (50.9%) had CD4 greater than 500 cells/mm3 and more than four-fifths of patients (82.7%) had undetectable level of viral load. TB was identified as one of the prevalent co-infections in the study area. Besides, nearly half of the patients were taking TB and pneumocystic carini pneumonia (PCP) prophylaxis concurrently during the first- and second-time changes. Studies reported that the majority of patients presented to the healthcare settings when the disease had become advanced (WHO clinical stage III).11,18,19,22 In concordant to our finding, study done in Tikur Anbesa Specialized Hospital (TASH) also indicated that the mean weight of the patients during initiation of ART was 55.6(±10) kg and four-fifths of patients had a CD4 count of less 200 cells/mm3 at the baseline.18
With respect to the duration of stay on initial ART regimen and trends of change, relatively higher number of patients had their initial regimen changed at the mid of the review period and the annual number of change declined almost to half through 2012 and 2013 and became steady thereafter. This might be partly related to the introduction of safer once-daily TDF-based regimen that created an opportunity for many patients to change to. D4 T related changes were found to be high around WHO official declaration in 2010.14,15 The majority of patients remained on initial ART regimens for 2–4 years (mean, 3.26 (±1.92) years). Niguse18 reported longer duration of stay on initial regimen at TASH where majority (82.6%) of the patients had been on ART for 5–10 years. However, the average durability of stay on first-time regimen, in this study, was found to be longer than the study conducted by De La Torre-lima et al.,23 where the mean duration was about 2.5 years. The longer duration of stay in TASH might be related to the presence of experienced specialists who have not permitted the change of regimens for some manageable reasons and let the regimen change only when the potential benefit for change outweighs the potential risk.
Majority of the patients were on d4T-based regimens with either NNRTIs (most of which were NVP containing) followed by those who were on AZT-based regimens. About one-tenths (10.45%) of patients started with TDF-based regimens. Concerning the distribution of initial regimen change, nearly two-thirds (66.36%) of patients had their initial regimen changed to TDF-based first-line and second-line regimens. Before the withdrawal of d4T, 30 (13.63%) patients had their initial regimen changed to d4T-based alternatives, and almost all of the changes were related to an interchange of either NNRTIs (NVP to EFV or vice versa). Few patients were subjected to change their regimen to ABC-based second-line alternative (ABC/3TC/LPV/r). Among the TDF-based regimens, nearly three-fourths of patients were transferred to EFV containing TDF-based regimen (TDF/3TC/EFV). Likewise, study done by Niguse18 indicated that about two-thirds (62%) of the patients were put on d4T-based regimens and few (4.0%) patients initiated their treatment with TDF. Several studies are in concordant with the present finding. The study done in Addis Ababa health facilities also indicated that the most common regimen before first switch was d4T-based (D4 T/3TC/NVP, 63% and D4 T/3TC/EFV, 18%).3 Comparably, Sivadasan et al.22 reported that the initial ART regimens used were 3TC with d4T (in 76%) or AZT and NVP (in 86%) or EFV. The study conducted in the Oromia Regional State further supported this study as the most common initial regimens were d4T/3TC/NVP (42.2%), d4T/3TC/EFV (27.5%), and AZT/3TC/EFV (12.7%).19 In contrary to this finding, study conducted by Dimala et al.11 at referral hospitals in Southwest Cameroon revealed that AZT/3TC/NVP regimen was received by majority (83%) of patients at the initial scenario. This might be due to the fact that authors included patients who initiated treatment very recently and hence the possibility of initiating with d4T-based regimen is unlikely. In line with our study, the above-mentioned studies reported that most of the patients had their initial regimen changed to TDF-based alternatives. Moreover, Deeks and Perry stressed that adherence to treatment was maintained or improved after switching to the once-daily triple combination, with patients generally preferring the TDF/FTC or 3TC/EFV single-tablet regimen over their previous more complex regimen. Thus, this single-tablet regimen provides a convenient once-daily regimen for use in treatment-experienced adults that may confer an advantage over more complex or frequently administered regimens for which adherence to treatment is an issue.14,15,24 However, TDF is associated with greater effect on decline in renal function and a higher risk of proximal tubular dysfunction in antiretroviral naive patients initiating TDF-based treatment.25
In our study, the three most frequently reported reasons for modifying the first-time regimens were found to be toxicity, comorbidity, and treatment failure with toxicities sharing around one-third (32.3%) of reasons for change. Among the toxicity-related changes, majority of which were attributable to rash (56.33%), hematologic disturbances (anemia) (19.72%), and CNS toxicities (15.49%). As regards to the treatment failure, about two-thirds (64.7%) of regimen changes were related to failure of immunological recovery (poor improvement in CD4 count). Besides, TB was identified as the major comorbid infection that complicated the treatment profile and increased the possibility of regimen change. The remaining changes were linked to adherence difficulty, pregnancy, stock-out, and undocumented reasons. Toxicities related to metabolic and hematologic disturbances (anemia and lipodystrophy) are attributable to NRTIs while NNRTIs accounted for CNS toxicities, rash, and hepatotoxicity. In Ethiopia, NVP and EFV have been remained as the only NNRTIs responsible for the aforementioned toxicities. Among the second-line regimens, protease inhibitors are commonly associated with lipid abnormalities and glucose intolerance (insulin resistance). However, there is a limited evidence to evaluate second-line therapies in patients with HIV who fail first-line treatment with a WHO-recommended regimen.26 It was pointed out that TDF was better tolerated with a substantially lower rate of drug substitutions due to adverse drug reactions.27 In the majority of studies, toxicity was most commonly reported reason for regimen modification.3,4,19,20,22,28,29 Regimen change could be an option for management of toxicities; however, it should be undertaken considering the risk of loss of future treatment options. Therapeutic drug monitoring may not be feasible in resource limited setting. Therefore, health providers working in the HIV clinic should monitor patients both clinically and with laboratory for the occurrence of side-effects.18,29 Studies also revealed that comorbidity was the second most common reason for regimen change.3,19 In this regard, TB was the main co-infection identified.
In 2010, WHO recommended the replacement of d4T by TDF in treatment protocols due to its associated metabolic side-effects.14 This change in guideline, alongside drug stock-outs, could be underlying reason for the extensive change in d4T-based regimens observed after 2010. However, this information could not be accurately ascertained from individual patient records, possibly overestimating the extent of stock-out and hence regimen changes.11 Therefore, we intentionally overlooked the reason for mere change of d4T in our study. Interference of WHO to ban d4T due to obvious toxicity reasons and high degree of stock-outs experienced thereafter forced us to exclude this drug alone. However, it does not mean that the whole d4T containing regimen was not considered.
Some studies also reported treatment failure as one of the major cause of regimen change.4,18,19,30 As per the study done by Haas et al.,30 58.0% of patients with confirmed virological and 19.3% of patients with confirmed immunological failure had their initial regimen changed within 2 years. Immunological success (improvement in CD4 count) was shown after regimen change. Undetectable viral loads were also measured in 83.9% patients post regimen change.20 Even if few patients had their initial regimen changed due to stock-outs and adherence issue, it might contribute its own share for the overall drug resistance and present a challenge to limited treatment options that we currently have for these patients.18,31 To this end, it is important is to maintain uninterrupted supplies of ART drugs in resource limited settings.32 Patient awareness about HIV/AIDS treatment should also be improved to solve adherence problems.
In this study, the prevalence of second-time regimen change was found to be 18.18%. With similar manner to initial regimen change, almost half of the patients (47.5%) had their initial regimen changed to TDF-based first- and second-line alternatives. However, no d4T-based regimen to be changed to since there was an official withdrawal. The three most commonly reported reasons for regimen change were toxicity (32.5%), treatment failure (27.5%), and pregnancy (17.5%). Finally, the type of ART regimens and TB treatment were found to have statistically significant association with the level of second-time regimen change. In line with our finding, Niguse reported 20.8% prevalence of second-time regimen change. In their study, during second-time changes, about one-third of the patients had their initial regimen change due to toxicity followed by poor adherence and treatment failure. Besides, the clinical stage and type of ART regimen were associated with the level of secondary change.18 What is more, having WHO clinical stage III, co-medication with ART, occurrence of TB, and side-effect on the regimen were independent predictors of regimen change.33
Strength and limitation
This study tried to address all aspects of regimen changes within 10 years review period. The study included comprehensive data on first- and second-time changes. It was also conducted with structured format customized from WHO guideline. The authors attempted to exclude d4T-specific regimen changes to avoid overestimation of reasons for regimen change in relation to unrecorded stock-outs of d4T after 2010. However, the study was not without potential limitations. It is a cross-sectional study that could not address future impacts and/or effectiveness of regimens. Since the sample size was relatively low, all eligible medical records were considered for the review. It is also a retrospective study in which some degree of documentation gap might be expected.
Conclusion
In this study, large proportion of patients experienced regimen change to TDF-based alternatives for both first- and second-time scenario. Majority of patients had got their regimens changed due to drug-related toxicities, treatment failure, and comorbid conditions. The most common toxicities experienced were rash, anemia, and CNS side-effects and were related to NVP, AZT, and EFV, respectively. Regimen change could be an option for management of toxicities and reducing the risk of treatment failure; however, it should be undertaken considering the risk of loss of future treatment options. The feasibility of therapeutic drug monitoring is unlikely in resource-limited setting. Therefore, health providers working in the HIV clinic should monitor patients both clinically and with laboratory for the occurrence of side effects and treatment failure. Some regimen changes were attributable to failure of either hospital supply system or patient-related factors which would have been prevented considering limited treatment options we currently have. It is recommended to have vivid and evidence-based risk–benefit analysis prior to changing ART regimens to ensure better quality and quantity of life years for patients.
Acknowledgments
The authors thank data collectors and JH staffs who assisted us a lot for the realization of this research.
Footnotes
Authors’ contribution: M.S., D.E., and M.G. conceived the study and drafted the proposal. All authors had substantial contribution in the study design and development of data collection checklist. All authors were also involved in data acquisition, analysis, interpretation and write-up. M.S. drafted the manuscript. D.E. and Y.A. reviewed it critically. M.S. also prepared the final draft for publication. All authors read and approved the final version of the manuscript.
Availability of data and materials: All the data used for the study are contained within the manuscript
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Proposal approval was obtained from Institutional Health Research Ethics Review Committee, Haramaya University. A letter of cooperation was obtained from College of Health and Medical Sciences (ref. no. C/AC/R/D/01/335/17) and submitted to Jugel Hospital.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Permission was obtained from the head of the hospital prior to the study. Written informed consent was not sought from the patient since the study did not directly involve HIV/AIDS patients who had been on ART follow-up. However, confidentiality of the patient information was maintained in such a way that the data abstraction format (checklist) was kept anonymous and data obtained from the hospital were solely used for the purpose of the study.
ORCID iD: Mekonnen Sisay  https://orcid.org/0000-0001-6611-1174
https://orcid.org/0000-0001-6611-1174
References
- 1. Wang H, Wolock TM, Carter A, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV 2016; 3(8): e361–e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birlie B, Braekers R, Awoke T, et al. Multi-state models for the analysis of time-to-treatment modification among HIV patients under highly active antiretroviral therapy in Southwest Ethiopia. BMC Infect Dis 2017; 17(1): 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jima YT, Angamo MT, Wabe NT. Causes for antiretroviral regimen change among HIV/AIDS patients in Addis Ababa, Ethiopia. Tanzan J Health Res 2013; 15(1): 11–18. [DOI] [PubMed] [Google Scholar]
- 4. Woldemedhin B, Wabe NT. The reason for regimen change among HIV/AIDS patients initiated on first line highly active antiretroviral therapy in Southern Ethiopia. N Am J Med Sci 2012; 4(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanaka H, Wada T, Takayama Y, et al. Evaluation of the efficacy and safety of changes in antiretroviral regimens for HIV-infected patients. J Pharm Pharm Sci 2014; 17(3): 316–323. [DOI] [PubMed] [Google Scholar]
- 6. Cicconi P, Cozzi Lepri A, Castagna A, et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naïve patients. HIV Med 2010; 11: 104–113. [DOI] [PubMed] [Google Scholar]
- 7. Cesar C, Shepherd BE, Krolewiecki AJ, et al. Rates and reasons for early change of first HAART in HIV-1-infected patients in 7 sites throughout the Caribbean and Latin America. PLoS ONE 2010; 5(6): e10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park WB, Choe PG, Kim SH, et al. Early modification of initial HAART regimen associated with poor clinical outcome in HIV patients. AIDS Res Hum Retroviruses 2007; 23(6): 794–800. [DOI] [PubMed] [Google Scholar]
- 9. Mocroft A, Phillips A, Soriano V, et al. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: increased incidence of stopping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Hum Retroviruses 2005; 21(9): 743–752. [DOI] [PubMed] [Google Scholar]
- 10. Bandeira Elias DBD, Cavalcante MG, Lima DGL, et al. Antiretroviral changes during the first year of therapy. Rev Assoc Med Bras 2017; 63(7): 606–612. [DOI] [PubMed] [Google Scholar]
- 11. Dimala CA, Bechem NN, Aroke D, et al. Motives for change of first-line antiretroviral therapy regimens in an unselected cohort of HIV/AIDS patients at a major referral centre in South-west Cameroon. BMC Res Notes 2017; 10(1): 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ndakala FN Oyugi JO OlukaKimani J et al. . The incidence of first-line antiretroviral treatment changes and related factors among HIV-infected sex workers in Nairobi, Kenya. Pan Afr Med J 2017; 28: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathai SCS. 2. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. June 2013. 3. Homsy J, Moore D, Barasa A, Likicho C, Waiswa B, et al. Medical Journal Armed Forces India 2013; 69: e412. [Google Scholar]
- 14. Doherty M, Ford N, Vitoria M, et al. The 2013 WHO guidelines for antiretroviral therapy: evidence-based recommendations to face new epidemic realities. Curr Opin HIV AIDS 2013; 8(6): 528–534. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization, 2016. [PubMed] [Google Scholar]
- 16. Bini T, Testa L, Chiesa E, et al. Outcome of a second-line protease inhibitor-containing regimen in patients failing or intolerant of a first highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2000; 24(2): 115–122. [DOI] [PubMed] [Google Scholar]
- 17. Ssozi J, Amlani S. The effectiveness of health expenditure on the proximate and ultimate goals of healthcare in Sub-Saharan Africa. World Development 2015; 76: 165–179. [Google Scholar]
- 18. Niguse H. Reasons for antiretroviral drug switch among patients attending at the antiretroviral therapy clinic of Tikur Anbesa Specialized Hospital, Addis Ababa, Ethiopia, 2016, http://etd.aau.edu.et/handle/123456789/2813
- 19. Wube M, Tesfaye A, Hawaze S. Antiretroviral therapy regimen change among HIV/AIDS Patients in Nekemt hospital: a primary care hospital in Oromia regional state, Ethiopia, 2013, http://www.japsonline.com/admin/php/uploads/993_pdf.pdf
- 20. Soorju V, Naidoo P. Confirmation of factors that influence antiretroviral regimen change and the subsequent patient outcomes at a Regional Hospital in rural KwaZulu-Natal. Afr J Prim Health Care Fam Med 2016; 8(1): e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bor J, Herbst AJ, Newell ML, et al. Increases in adult life expectancy in Rural South Africa: valuing the Scale-Up of HIV treatment. Science 2013; 339(6122): 961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sivadasan A, Abraham O, Rupali P, et al. High rates of regimen change due to drug toxicity among a cohort of South Indian adults with HIV infection initiated on generic, first-line antiretroviral treatment. J Assoc Physicians India 2009; 57: 384–388. [PubMed] [Google Scholar]
- 23. De La, Torre-Lima J, Aguilar A, Santos J, et al. Durability of the first antiretroviral treatment regimen and reasons for change in patients with HIV infection. HIV Clin Trials 2014; 15(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 24. Deeks ED, Perry CM. Efavirenz/emtricitabine/tenofovir disoproxil fumarate single-tablet regimen (Atripla®). Drugs 2010; 70(17): 2315–2338. [DOI] [PubMed] [Google Scholar]
- 25. Horberg M, Tang B, Towner W, et al. Impact of tenofovir on renal function in HIV-infected, antiretroviral-naive patients. J Acquir Immune Defic Syndr 2010; 53(1): 62–69. [DOI] [PubMed] [Google Scholar]
- 26. Humphreys EH, Chang LW, Harris J. Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst Rev 2010; 6: CD006517. [DOI] [PubMed] [Google Scholar]
- 27. Njuguna C, Orrell C, Kaplan R, et al. Rates of switching antiretroviral drugs in a primary care service in South Africa before and after introduction of tenofovir. PLoS ONE 2013; 8(5): e63596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torres TS, Cardoso SW, Velasque LS, et al. Incidence rate of modifying or discontinuing first combined antiretroviral therapy regimen due to toxicity during the first year of treatment stratified by age. Braz J Infect Dis 2014; 18(1): 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Teklay G, Legesse B, Legesse M. Adverse effects and regimen switch among patients on antiretroviral treatment in a resource limited setting in Ethiopia. J Pharmacovigilance 2013; 1: 115. [Google Scholar]
- 30. Haas AD, Keiser O, Balestre E, et al. Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV 2015; 2(7): e271–e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nevin CR, Ye J, Aban I, et al. The role of toxicity-related regimen changes in the development of antiretroviral resistance. AIDS Res Hum Retroviruses 2011; 27(9): 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schouten EJ, Jahn A, Ben-Smith A, et al. Antiretroviral drug supply challenges in the era of scaling up ART in Malawi. J Int AIDS Soc 2011; 14(Suppl. 1): S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anlay DZ, Alemayehu ZA, Dachew BA. Rate of initial highly active anti-retroviral therapy regimen change and its predictors among adult HIV patients at University of Gondar Referral Hospital, Northwest Ethiopia: a retrospective follow up study. AIDS Res Ther 2016; 13: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]