Abstract
Background:
Cyclophosphamide (CP), as a chemotherapy drug, causes severe damage in testicular tissue through producing free radicals. Cerium oxide nanoparticles (NC) exhibit antioxidant and anti-inflammatory properties. The purpose of this study was to investigate the protective effect of NC on CP-induced testicular damage in mice.
Methods:
In this experimental study, thirty-two male mice were divided into four groups (eight mice in each group). The control group was received intraperitoneally (IP) normal saline, NC group was received NC for three consecutive days (100 μg/kg, IP), CP group was received CP (200 mg/kg, IP), and the CP + NC group received NC, three consecutive days before receiving CP. After 2 days, testicles were assessed for biochemical, histomorphometrical, histopathological, and immunohistochemical analyses.
Results:
CP administration caused statistically significant increases in sperm abnormality, malondialdehyde, protein carbonyl levels, reactive oxygen species, level and apoptosis, and decreases in sperm count, sperm viability, testosterone, glutathione activity, the mean thickness of the germinal epithelium, diameter of seminiferous tubules in mice. Degeneration, necrosis, arrest of spermatogenesis, congestion, and atrophy in testicular tissue confirmed the low Johnsen's Testicular score in CP group. Administration of NC significantly ameliorated the CP-induced adverse effects on testis compared with the CP group. In addition, pretreatment mice with NC significantly reduced caspase-3 immunoreactivity induced by CP in testis.
Conclusions:
This study showed that NC with scavenging free radicals and antiapoptotic properties enable to reduce the side effects of CP in the testicular tissue.
Keywords: Caspase-3, cerium oxide, cyclophosphamide, oxidative stress, testis, toxicity
Introduction
Cyclophosphamide (CP), a cytotoxic alkylating agent, is one of the chemotherapeutic drugs that is widely used in the treatment of cancers and also is used as an immunosuppressive drug in organ transplantation and autoimmune diseases.[1] Side effects of CP are testicular weight loss, seminiferous tubules (ST) atrophy, oligospermia, azoospermia, decrease of testosterone, spermatogenic cells degeneration, and apoptosis.[1,2] CP induces biochemical and histological alterations through producing oxidative stress in cells.[3] The production of free radicals disrupts the balance of antioxidants and pro-oxidants and results in tissue injury.[4] Several studies have been shown that exogenous antioxidants to reduce oxidative damage arising from CP.[5,6]
Cerium oxide (CeO2) nanoparticle (nanoceria, NC), a rare earth element in the lanthanide series, in the form of trivalent (Ce3+) and quadrivalent (Ce4+) state allows nanoparticles to store and release oxygen.[7] NC recently has remarkable therapeutic applications. This nanoparticle with antioxidant property react with superoxide and hydrogen peroxide and similar two endogenous antioxidant enzymes, such as superoxide dismutase and catalase, scavenge intracellular reactive oxygen species (ROS).[8] Previous studies demonstrated the antioxidant,[9] anti-inflammatory,[10] and anti-angiogenesis[11] properties of NC. NC enables to protect tissue against side effects induced by radiotherapy,[12] cardiomyopathy,[13] chemotherapy,[14] and ischemia.[15] However, other studies revealed that NC with production of ROS and creating oxidative stress exhibited cellular toxicity.[16,17] So far no study has investigated the protective effect of NC against CP-induced testicular toxicity.
Here, we describe the biological effects of NC in testicular injury induced by CP in mice model. Oxidative stress generated by CP plays a significant role in testicular injury. According to the above, it has been hypothesized that NC by having antioxidant properties can improve oxidative stress-induced testicular toxicity. In this study we assessed the protective effects of NC on testicular toxicity against CP by evaluating biochemical, immunohistochemical, and histological parameters.
Materials and Methods
Chemicals
ELISA Kit was obtained from Bioassay Company for testosterone assay (China). CP was purchased from Baxter Company (Germany). CeO2 nanoparticle was obtained from Neutrino Co. (Iran). Particle size was 378 nm, with polydispersity index (PDI) 0.372 and zeta-7.11 that were determined with Zetasizer 3600 Nano ZS (Malvern Instrument Ltd, Malvern, UK).
Animals
All the animal experiments and research methods were approved by the Institutional Animal Care and Ethics Committee of the Medical Science of Mazandaran University, Sari, Iran (ID: IR.MAZUMS.REC.1395.2575). Thirty-two male BALB/c mice (25–30 g) were maintained on a 12:12 dark/light cycle and 20°C–25°C environment. They had free access to food and water during the study period. They were allowed 1 week to acclimate to the experiment environment.
Study design
Animals were randomly divided into four experimental groups (eight mice in each group): Group I: Mice received normal saline (same volume with other groups) as a vehicle of NC for 3 days (no-treated CeO2 or CP).
Group II: Mice received 100 μg/kg NC daily intraperitoneally (IP) for three consecutive days.
Group III: Mice received 200 mg/kg CP IP single dose.
Group IV: Mice received NC daily for three consecutive days and on the third day, mice were received CP.
The dosage and the number of days of receiving the NC were selected on the basis of pilot study. In high doses of NC, some animals died and/or testicular tissue was seriously destroyed. Nanoparticles were freshly suspended in normal saline solution. Nanoparticles were vortexed just before administration in a volume of 0.2 mL. In NC + CP group, NC was administered 1 hour before CP treatment. The fifth day of the study, biochemical, histological, and immunohistochemical assays were evaluated.
Specimen collection
Two days after drug administration, the animals were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg). Blood samples were collected from the heart. Serum was separated from blood with 15 min of centrifugation at 3000 ×g, and stored at −20°C for evaluate testosterone analysis. Then animals were sacrificed and right testis was immediately removed, washed with phosphate-buffered saline (PBS) and weighed. For biochemical evaluation, samples were freshly used. The left testis was removed and fixed in 10% buffer formalin for histological and immunohistochemical assay.
Sperm collection
For evaluation sperm parameters, the epididymal tissue removed from testis, minced into small plate containing 1 mL preheated Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum. After 15 min incubation, the cell suspension was used for sperm parameters analysis including percentage of sperm motility, total sperm count, sperm viability, and determining percentage of sperm morphology.
Evaluation of sperm parameters
For sperm count, 20 μL of sperm suspension was placed on Neubauer hemocytometer and at a magnification of ×40 counted.
To determine the percentage of sperm motility, one drop of the sperm suspension was placed on the slide, and the percentage of sperm motility according to the type move was appraised using a light microscope with a magnification of ×10 in three different fields for each sample, and the percentage of sperms with normal and abnormal motility in the fields was recorded. Then average numbers were considered as the final motility score.[18]
To determine the percentage of sperm morphology, one drop of 10 μL of the sperm suspension with 10 μL of eosin mixed. After 1 min incubation, smear prepared with a drop of 12 μL onto a glass slide. After drying, sperm morphology checked. Abnormal shape of the head and tail of sperm analyzed in the prepared slides and mean data were recorded.
To assess sperm viability 20 μL of sperm suspensions with 20 μL of 1% eosin-Y were mixed and after 3–4 min stained and unstained cells were counted using Neubauer hemocytometer with ×40 magnification inverted microscope. Each sample was measured at least three times.
Mitochondrial preparation of testicular tissues
After homogenization and centrifugation of fresh testicular tissues, mitochondrium were separated. Testes was removed and minced in a cold mannitol (225 mM) solution and then homogenized and centrifuged (1500 ×g, 10 min) at 4°C. Supernatant was removed for further centrifugation (11,000 ×g, 10 min) and then supernatant was discarded. The sedimented mitochondrial pellet was gently washed and then suspended in the isolation medium (0.225 MD-mannitol, 75 mM sucrose, and 0.2 mM EDTA, pH 7.4) and then centrifuged again (11,000 ×g for 10 min). Final mitochondrial sample were suspended in Tris buffer. For assessment, the ROS generation in mitochondria, it was used as a respiration buffer (0.32 mM sucrose, 10 mM Tris, 20 mM Mops, 50 μM EGTA, 0.5 mM MgCl2, 0.1 mM KH2PO4, and 5 mM sodium succinate). Mitochondria were freshly prepared for each experiment and were used within 4 h of isolation. The mitochondrial samples (0.5 mg mitochondrial protein/mL) were used in this study.
Protein concentration
Concentration of protein in mitochondria was measured by the Coomassie blue protein binding method. Bovine serum albumin was used as the standard for evaluation of the protein content.
Biochemical analysis
ROS assay in mitochondria
ROS levels of mitochondria were measured using the DCFH-DA reagent. Isolated testicular mitochondria (0.5 mg mitochondrial protein/mL) were placed in respiration buffer and then 20 μL DCFH-DA was added to the samples (final concentration, 10 μM) and incubated at 37°C for 15 min. Then absorption was measured by fluorescence spectrophotometer (Shimadzu RF5000U) at the λex = 488 nm and λem = 527 nm.
Evaluation of malondialdehyde formation in mitochondria
The concentrations of testicular lipid peroxidation were measured by estimating of malondialdehyde (MDA) using the thiobarbituric acid (TBA) with a spectrophotometric assay. To begin the analysis, 0.25 mL phosphoric acid (0.05 M) was mixed to the 0.2 mL of sample and then 0.3 mL of 0.2% TBA was added. Samples were reserved in a boiling water bath for 30 min. The sample tubes were placed to the ice-bath and then 0.4 mL of n-butanol was added to each sample. Then were centrifuged (3500 rpm) for 10 min and MDA was measured based on reaction with TBA (an MDA–TBA complex). Created MDA in each sample was calculated in the supernatant at 532 nm with ELISA reader (Tecan, Rainbow Thermo, Austria). MDA content was expressed as μg/mg protein. Tetramethoxypropane (TEP) was used in this experiment as standard.
Evaluation of protein carbonyl content in mitochondria
The protein carbonyl (PC) was measured by using 2,4-dinitrophenyl-hydrazine (DNPH) reagent. After determination of tissue protein, 500 μL of trichloroacetic acid (20% w/v) was added to the sample and stored at 4°C for 15 min. Then precipitated protein was centrifuged at 6500 ×g for 10 min and the supernatant was discarded. Soluble protein (0.5 mL) was reacted with DNPH 10 mM (0.5 mL) in HC1 2 M for 1 h at room temperature. Precipitate was washed with 1 mL mixture of ethanol and ethyl acetate 1:1 (v/v) and then centrifuged at 6500 ×g for 10 min and the supernatant was removed. The final protein deposition solubilized in 200 μL Guanine hydrochloride solution and was centrifuged at 16,000 ×g for 5 min to remove any trace of insoluble material. The protein carbonyl were assessed spectrophotometrically by reading the absorption at a wavelength of 365 nm with an absorption coefficient of 22,000 M−1 cm−1 was expressed as a nmol of DNPH per milligram of protein.
Measurement of glutathione content
Content of the glutathione (GSH) in the samples was determined by spectrophotometer (UV-1601 PC, Shimadzu, Japan) with 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) as an indicator at 412 nm and expressed as μM.
Testosterone assay
Levels of testosterone in serum were determined by using radioimmunoassay according to manufacturer's instructions (Mouse Testosterone ELISA Kit, Bioassay, Cat. No. E0260MO). Testosterone amount is expressed as nmol/L. All samples were carried in duplicate.
Histopathological assay
For microscopic evaluation and to determine the effect of CP and nanoceria in testis, the tissue samples were fixed in 10% buffer formalin. After fixation, processing, and embedding in paraffin, sections with 5 μM thickness stained with hematoxylin and eosin (H and E) were investigated under a light microscope (Olympus, Japan). Testicular tissue structure was investigated with Johnsen's scoring (JS) system, five sections per animal and ten ST per section were assessed using a score of 1–10 under ×40 magnification.[19]
Histomorphometric assay
For quantitative evaluation, the average diameter of ST and thickness of the germinal epithelium of the ST (from the basement membrane to lumen)[20] in ten tubule per testicular section and ten section per groups were measured at ×40 magnifications by using calibrated OLYSIA Soft Imaging System GmbH, version 3.2 (Japan). All specimens were evaluated by a histologist as blind.
Immunohistochemical assay
Immunohistochemical technique was performed according to the instructions kit manufacturer (Abcam Company, USA). After deparaffinization with xylene and rehydration in alcohol series, endogenous peroxidase activity was blocked by 0.3% H2O2 in methanol by incubating the sections for 15 min. Then, tissue sections were incubated at 4°C overnight with primary antibodies (anti-caspase-3 rabbit polyclonal antibody, 1:100 in PBS, v/v, Abcam, lat: GR224831-2). After incubating with secondary antibody conjugated with horseradish peroxidase (Mouse and Rabbit Specific HRP/DAB, Abcam, Lat: GR2623314-4) for 15 min, sections were incubated with diaminobenzidine tetrahydrochloride for 5 min.[10] Then, the samples were dehydrated and mounted. The primary antibody was omitted for negative controls. For the quantitative analysis, immunohistochemical photomicrographs were evaluated by densitometry using ImageJ software (MacBiophotonics ImageJ, 1.41a version). The positive staining severity was assessed as the ratio of the stained area to the entire field assessment.
Statistical analysis
Data were analyzed by SPSS software, (version 19, Chicago, USA), and Prism software (GraphPad, version 6.07, USA). All of the data with normally distributed are presented as the mean ± standard deviation (M ± SD). One-way analysis of variance and Tukey tests were used. Statistically significant differences were accepted as P < 0.05.
Results
Oxidative stress biomarkers
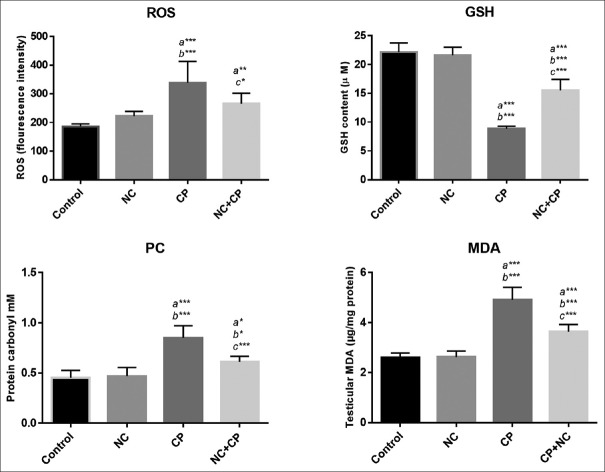
MDA, GSH, and PC levels as indicators of oxidative stress in testis are presented in Figure 1. Intracellular ROS formation, MDA, and protein carbonyl contents significantly increased (P < 0.001) in CP-treated group when compared with control group. Administration of NC at dose of 100 μg/kg significantly decreased the elevated ROS in NC + CP group compared to CP group. No significant changes were observed in ROS level in animals that received only NC compared with control group.
Figure 1.
Effect of NC against CP-induced testicular damages on ROS, MDA, GSH, and protein carbonyl levels. All values are expressed as mean ± SD. a significant vs. control, b significant vs. NC, and c significant vs. CP groups. *P < 0.05, **P < 0.01, ***P < 0.001. NC; nanoceria, CP; cyclophosphamide.
In CP group, the GSH significantly (P < 0.001) was decreased compared with the control and NC groups; an impaired antioxidant system in CP-treated mice. While disruption of the endogenous antioxidant system was significantly increased (P < 0.001) in animals that were treated with NC and CP.
Sperm parameters
Sperm parameter findings in all the groups are presented in Table 1. Nanoceria treatment had no effect on sperm parameters. CP administration caused significantly increased abnormal sperm rate (P < 0.01) and decreased sperm count (P < 0.001), viability rate (P < 0.01), and sperm motility (P < 0.001) compared with the NC and control group. NC treatment in CP-treated mice could significantly (P < 0.05 and P < 0.01) improve sperm parameters except sperm abnormality compared to the CP group.
Table 1.
Sperm count, abnormality, viability rate, and motility, epithelial thickness (ET), seminiferous tubules diameter (SD), and serum testosterone level in the all groups
| Groups | Control | NC | CP | CP + NC |
|---|---|---|---|---|
| Sperm count (×106) | 8.86±0.9 | 9.05±0.92 | 3.05±0.6a,b*** | 4.86±0.96a,b,c** |
| Sperm abnormality (%) | 7.8±2.95 | 9.2±2.86 | 19.6±6.35a,b** | 14.2±3.42 |
| Sperm viability (%) | 71.3±4.33 | 73.84±3.89 | 48.88±13.97a,b*** | 63.71±4.94c* |
| Sperm motility (%) | 89.6±7.02 | 87.4±6.11 | 49.6±8.26a,b*** | 68.6±6.58a,b,c** |
| ET (µm) | 76.48±7.45 | 73.86±7.22 | 42.72±7.75a,b*** | 61.53±8.3a,b,c*** |
| SD (µm) | 203.7±14.56 | 198.1±14.82 | 187.7±17.44a,b*** | 196.5±15.91a,c** |
| Testosterone (nmol/L) | 14.02±2.61 | 11.69±1.56 | 4.33±2.36a,b*** | 9.65±1.17a,c** |
All values are expressed as mean±SD. asignificant vs. control, bsignificant vs. NC, and csignificant vs. CP groups. *P<0.05, **P<0.01, and ***P<0.001. NC=Nanoceria, CP=Cyclophosphamide.
Testosterone level
Serum testosterone levels are presented in Table 1. Serum testosterone levels were significantly decreased in CP group compared with the control group (P < 0.001). Administration of NC in CP-treated mice significantly increased serum testosterone level (P < 0.01).
Histopathological findings
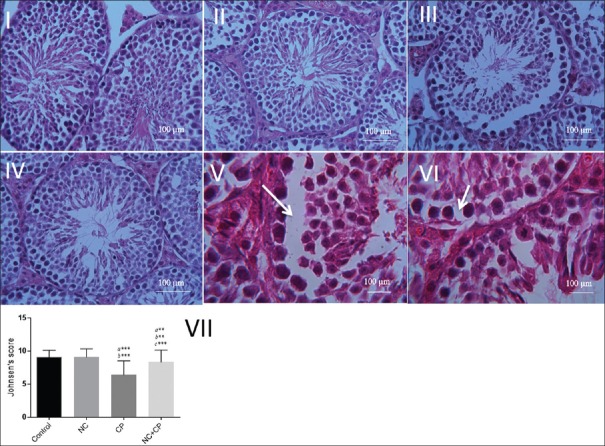
The photomicrographs of testicular sections in all the groups are presented in Figure 2. Normal spermatogenic cells (spermatogonia, spermatocyte, and spermatid), somatic testicular cells (sertoli and leydig cells), and accurate spermatogenesis with abundant spermatids were observed in control group [Figure 1a]. These histopatholoical parameters were similar in mice treated with NC and control group [Figure 2b]. Testis sections of mice treated with CP revealed many adverse histological changes. These include disorganization in the epithelial layer of ST, detachment (desquamation) of spermatogenic cells, especially spermatogonia, spermatocytes, and spermatid in ST, degenerative cells, edema, and congestion, vacuolization in spermatogonia and sertoli cells, Leydig cell destruction, and lipid granules in cytoplasm of the Leydig cells [Figure 2c, e and f]. Whereas testis of mice treated daily with NC for 3 days before CP administration exhibited mild degeneration of ST with less structural changes in spermatogenic cells, mild congestion, edema, and the integrity of interstitial tissue was preserved [Figure 2d]. Low Johnsen's score were observed in the CP groups. Scores of testes treated with NC + CP was higher compared to the CP group (P < 0.05) [Figure 2g].
Figure 2.
Photomicrographs showed the effect of NC on the histological architecture of testis in the groups. Control (I), NC (II), CP (III, V, VI), and NC + CP (IV) groups. Normal structure in control group, detachment (V), vacuolization (VI) in the seminiferous tubules in CP group. (H and E staining, Mag. (I, II, III, IV) ×40, (V, VI) ×100. Scale bar = 100 μm. (VII) Johnsen's score in testicular tissue. Data are presented as mean ± SD. a significant vs. control, b significant vs. NC, and c significant vs. CP groups. **P < 0.01, ***P < 0.001. NC; nanoceria, CP; cyclophosphamide.
Histomorphometrical findings
Morphometric findings in CP-treated group showed reduced seminiferous epithelial thickness and atrophy of seminiferous tubules in numerous tubules as compared to control (P < 0.001). NC administration increased the mean germinal epithelium thickness (P < 0.001) and tubular diameter (P < 0.01) in CP-treated mice. These changes in thickness of the epithelium and diameter of ST were statistically significant compared to the CP mice (P < 0.001) and (P < 0.01), respectively [Table 1].
Immunohistological findings
Results of immunohistological staining of the testes are shown in Figure 3. Section of testes showed a low level caspase-3 immunoreactivity in the control group. Immunoreactivity level of caspase-3 in the NC group was similar to control group. Section of testis tissues in CP-treated mice displayed increase immunoreactivity level of caspase-3. Immunoreactivity mainly localized in the spermatogonia cells and less in the stromal cells [Figure 3a] of the testis. While NC + CP group showed mild immunoreactivity level of caspase-3 in the spermatogonial cells [Figure 3b] compared to CP-treated testis.
Figure 3.
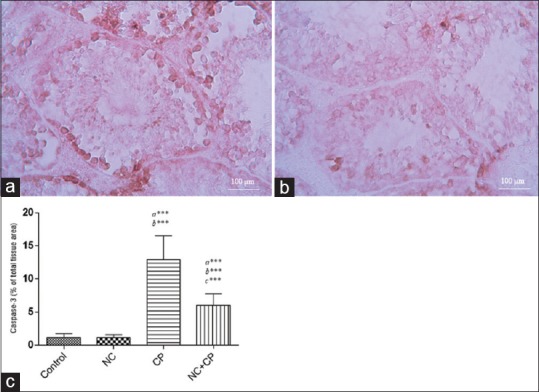
(a) Immunohistochemical staining demonstrated the caspase-3 immunoreactivity in CP group that were remarkable in spermatogonia cells and weaker in spermatocyte cells. (b) NC treatment diminished caspase-3 immunoreactivity in CP-treated mice. Mag; ×40. Scale bar = 100 μm. (c) Densitometry analysis of immunohistochemical staining for caspase-3. Data were presented as a percentage of total tissue area. Immunoreactivity level of caspase-3 in the control group was similar to NC alone groups. a significant vs. control, b significant vs. NC, and c significant vs. CP groups. ***P < 0.001.
The histograms of the semi-quantitative analysis of caspase-3 staining in all groups are shown in Figure 3c. The most intense immunoreactivity of caspase-3 was confirmed by semi-quantitative analysis in CP-treated mice (12.94 ± 3.6) compared with the other groups (P < 0.001). NC treatment decreased severity of immunoreactivity of caspase-3 (6.03 ± 1.73). Level of caspase-3 in the control group (1.18 ± 0.55) was similar to NC group (1.16 ± 0.44).
Discussion
In this study, at the first time, the effect of NC on CP-induced testicular toxicity was investigated. The present study showed protective effect of NC on CP-induced testicular damage. The results revealed that NC improved the reduced serum testosterone level, sperm count, and abnormal sperm in CP-treated animals. It also decreased lipid peroxidation, ROS, and PC levels. Moreover, administration of NC exhibited mitigated apoptosis in the testis. Its protective mechanisms may be related to its anti-oxidative and anti-apoptotic properties of NC.
Oxidative stress is caused by an imbalance in the production and absorption capacity of ROS in cells that subsequently leading to tissue damage.[21] It has been revealed that oxidative stress by inactivating microsomal enzymes increase ROS and lipid peroxidation.[22] CP with production of free radicals and reduction of antioxidants is involved in oxidative stress.[23] Increase in testicular ROS level was stated in CP-treated animals.[24] The biochemical analysis in this study confirmed that decreased antioxidant enzyme like GSH and increased MDA level in the testis showed oxidative stress and is consistent with previous study.[25]
NC biologically catalyze electron transfer reactions, such as superoxide dismutase, catalase, and glutathione.[15] The NC scavenged ROS produced in testis. We observed significantly lower levels of ROS, lipid peroxidation, and PC in NC + CP group than in the CP group. We also observed significantly higher levels of GSH in NC + CP group than in the CP group. It was reported previously that NC by imitating of superoxide dismutase and catalase scavenge ROS.[8]
It was reported that NC induced oxidative stress in both in vitro[26] and in vivo.[13] The toxic effect of NC is associated with increased oxidative stress.[16,17] On the other hand, the antioxidant effect of NC plays a protective role in the body by eliminating free oxygen radicals.[27] The above suggests that there are still some disagreements over NC toxicity. Niu and colleagues showed treatment with NC specifically inhibited infiltration of monocytes/macrophages, apoptotic cell death, and expression of proinflammatory cytokines TNF-α, IL-1β, and IL-6.[13] Also, Sayes and colleagues reported ROS production and apoptotic cell death have been found in cancer cells with NC treated.[28] Different biological effects of NC have been attributed to factors such as construction method, particle size, purity, and surface charge.[29] In this study, we used nanoceria with size of 378 nm, PDI of 0.372 and zeta of 7.11 that could have antioxidant property and protective effect on testicular damage induced by CP.
Sperm is very sensitive to ROS due to high concentrations of polyunsaturated fatty acids and low antioxidant capacity.[30,31] Obviously, lipid peroxidation of sperm leads to destruction in spermatozoa membranes that is associated with a sharp drop of intracellular ATP, reduction of sperm viability and increased morphological defects, and even impaired spermatogenesis.[3] Also, t was revealed that ROS and oxidative stress has a significant association with decreased sperm motility.[32] In this study, CP increased the number of abnormal sperm, and decreased sperm counts, sperm viability, and motility. Also, it was seen that NC treatment in CP-treated mice increased significantly the sperm viability and motility, and decreased the number of abnormal sperms, but it was non-significant. So, due to the reduction of ROS, it may be related to antioxidant property of NC that is involved in the protection of sperm cells in CP-treated animals.
The nanoparticles play a role in the sperm-mediated gene transfer technique (SMGT) for the transfer of sperm DNA into oocyte.[33] Falchi et al. showed that sperm cells exposed to NC for 24 h in vitro, NC does not penetrate inside the cell, and has no effect on the sperm motility. Also, there is no cytotoxicity and genotoxicity on sperm cells.[34] In this study, in vivo model, NC did not have any toxic effects on the sperm.
Histopathological assay revealed CP causes disorganization of ST, degeneration of spermatogenic cells, and vacuolation in spermatogonia, spermatocytes, and reduction in germ cells, arrest of spermatogenic maturation, vacuolization in spermatogonia and sertoli cells, interstitial edema, and congestion. Also, the mean JS in CP group was determined a lower value compared to control group. Damage in testicular tissue structure may be attributed that CP induces lipid peroxidation, oxidative stress, and subsequently disruption in the structure and function of testis. These findings are consistent with the results of others.[33] In the present study, administrations of NC to CP-treated mice showed an amelioration effect in the testicular tissue structure compared with the CP group.
Histomorphometric assay revealed reducing the diameter of the ST and thickness of the germinal epithelium in CP treated as indicated by reducing the number of spermatogenic cells. Further, CP treatment is leading to decrease serum testosterone. Administration of NC ameliorated these changes in testis. Increase in oxidative stress and ROS induced damage to DNA, protein, and enzymes that causes Leydig cell degeneration and disruption in steroidogenesis and spermatogenesis.[35] Furthermore, lipid peroxidation and decrease in plasma testosterone level are associated with reduced 3b-hydroxysteroid dehydrogenase (3b-HSD) and 17b-hydroxysteroid dehydrogenase (17b-HSD), the key enzymes that play a role in regulation of the production of testosterone.[36] In the present study, serum testosterone level reduced and spermatogenesis disrupted. Decrease of serum testosterone level after CP treatment is aligned with other study.[37] In ELISA examination, our results showed that NC elevated testosterone levels. These improvements in histomorphometric and histopathologic findings after NC administrations may be attributed to free radical scavenging effect of these nanoparticles.
The toxic effect of chemotherapy is directly on Leydig cells and indirectly on spermatogenic cells.[38] For biosynthesis of steroid hormones, neutral lipids accumulate in the spermatogenic cells. In the CP treated, accumulation of unconsumed lipids in the spermatogenic cells destroy spermatogenic cells.[23] On the other hand, this lipid accumulation because of phagocytosis of the apoptotic spermatogenic cells may also be seen by sertoli cells.[39]
NC by having anti-apoptotic and antioxidant properties has a protective effect on oxidative stress-induced damage.[12] This is in line with our study that CP-induced apoptosis confirmed by immunohistochemistry and was subsequently decreased by NC intervention. Colon and colleagues reported that administration of NC IP 24 h before irradiation protect gastrointestinal epithelium injury induced irradiation by reducing the amount of ROS, decreased caspase-3, and increased superoxide dismutase 2 expression.[12] The high apoptosis is associated with high ROS production derived from cytotoxic CP treatment.[40] Oxidative stress has been shown to promote apoptosis through the activation of the caspase-3[41] that is a major cause of infertility. In the present study, CP was significantly increased caspase-3 immunoreactivity in the testes that confirms the association of oxidative stress with apoptosis. Also, immunohistochemistry analysis demonstrated apoptotic cells and caspase-3 immunoreactivity were significantly reduced by NC treatment. The anti-apoptotic property of NC is consistent with other study.[12]
Conclusions
In conclusion, the present study showed the protective effect of NC against chemotherapy-induced oxidative stress. The results of this study proved NC protect CP-induced testicular damage via anti-oxidative and anti-apoptotic properties. NC down-regulates caspase-3 and reduces ROS, lipid peroxidation, and protein carbonyl. These findings suggest that the therapeutic potential of engineered NC may be a good candidate for treatment of oxidative injury induced by chemotherapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research was supported by the Molecular and Cell Biology Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
References
- 1.Türk G, Çeribaşi AO, Sakin F, Sönmez M, Ateşşahin A. Antiperoxidative and anti-apoptotic effects of lycopene and ellagic acid on cyclophosphamide-induced testicular lipid peroxidation and apoptosis. Reprod Fertil Dev. 2010;22:587–96. doi: 10.1071/RD09078. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar R, Miller SD, Meyers KE, Ginsberg JP. Fertility preservation in patients receiving cyclophosphamide therapy for renal disease. Pediatr Nephrol. 2015;30:1099–106. doi: 10.1007/s00467-014-2897-1. [DOI] [PubMed] [Google Scholar]
- 3.Çeribaşi AO, Türk G, Sönmez M, Sakin F, Ateşşahin A. Toxic Effect of Cyclophosphamide on Sperm Morphology, Testicular Histology and Blood Oxidant-Antioxidant Balance, and Protective Roles of Lycopene and Ellagic Acid. Basic Clin Pharmacol Toxicol. 2010;107:730–6. doi: 10.1111/j.1742-7843.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 4.Ray S, Pandit B, Das S, Chakraborty S. Cyclophosphamide-Induced Lipid Peroxidation and Changes in Cholesterol Content: Protective Role of Reduced Glutathione. Iranian J Pharm Sci. 2011;7:255–67. [Google Scholar]
- 5.Chabra A, Shokrzadeh M, Naghshvar F, Salehi F, Ahmadi A. Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum Exp Toxicol. 2014;33:185–95. doi: 10.1177/0960327113489052. [DOI] [PubMed] [Google Scholar]
- 6.Abarikwu S, Otuechere C, Ekor M, Monwuba K, Osobu D. Rutin ameliorates cyclophosphamide-induced reproductive toxicity in male rats. Toxicol Int. 2012;19:207. doi: 10.4103/0971-6580.97224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skorodumova N, Simak S, Lundqvist BI, Abrikosov I, Johansson B. Quantum origin of the oxygen storage capability of ceria. Phys Rev Lett. 2002;89:166601. doi: 10.1103/PhysRevLett.89.166601. [DOI] [PubMed] [Google Scholar]
- 8.Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3:1411–20. doi: 10.1039/c0nr00875c. [DOI] [PubMed] [Google Scholar]
- 9.Zhai Y, Zhang Y, Qin F, Yao X. An electrochemical DNA biosensor for evaluating the effect of mix anion in cellular fluid on the antioxidant activity of CeO2 nanoparticles. Biosens Bioelectron. 2015;70:130–6. doi: 10.1016/j.bios.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, Reilly CM. Anti-inflammatory Properties of Cerium Oxide Nanoparticles. Small. 2009;5:2848–56. doi: 10.1002/smll.200901048. [DOI] [PubMed] [Google Scholar]
- 11.Das S, Singh S, Dowding JM, Oommen S, Kumar A, Sayle TX, et al. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33:7746–55. doi: 10.1016/j.biomaterials.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6:698–705. doi: 10.1016/j.nano.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549–59. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamzeh M, Amiri FT, Beklar SY, Hosseinimehr SJ. Nephroprotective effect of cerium oxide nanoparticles on cyclophosphamide-induced nephrotoxicity via anti-apoptotic and antioxidant properties in BALB/c mice. Marmara Pharm J. 2018;22 [Google Scholar]
- 15.Estevez A, Pritchard S, Harper K, Aston J, Lynch A, Lucky J, et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radical Biol Med. 2011;51:1155–63. doi: 10.1016/j.freeradbiomed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Auffan M, Rose J, Orsiere T, De Meo M, Thill A, Zeyons O, et al. CeO2 nanoparticles induce DNA damage towards human dermal fibroblasts in vitro. Nanotoxicology. 2009;3:161–71. [Google Scholar]
- 17.Park E-J, Choi J, Park Y-K, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245:90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Aksu E, Kandemir F, Özkaraca M, Ömür A, Küçükler S, Çomaklı S. Rutin ameliorates cisplatin-induced reproductive damage via suppression of oxidative stress and apoptosis in adult male rats. Andrologia. 2017;49 doi: 10.1111/and.12593. [DOI] [PubMed] [Google Scholar]
- 19.Naeimi RA, Amiri FT, Khalatbary AR, Ghasemi A, Zargari M, Ghesemi M, et al. Atorvastatin mitigates testicular injuries induced by ionizing radiation in mice. Reprod Toxicol. 2017;72:115–21. doi: 10.1016/j.reprotox.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 20.Mirhoseini M, Amiri FT, Malekshah AAK, Gatabi ZR, Ghaffari E. Protective effects of melatonin on testis histology following acute torsion-detorsion in rats. Int J Reprod Biomed. 2017;15:141. [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan T, Cong Y, Meng J, Qian H, Ye W, Sun W-S, et al. Arachidonic acid causes hidden blood loss–like red blood cell damage through oxidative stress reactions. J Surg Res. 2017;211:14–20. doi: 10.1016/j.jss.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 22.Lear L, Nation R, Stupans I. Effects of cyclophosphamide and adriamycin on rat hepatic microsomal glucuronidation and lipid peroxidation. Biochem Pharmacol. 1992;44:747–53. doi: 10.1016/0006-2952(92)90412-c. [DOI] [PubMed] [Google Scholar]
- 23.Bakhtiary Z, Shahrooz R, Ahmadi A, Soltanalinejad F. Ethyl pyruvate ameliorates the damage induced by cyclophosphamide on adult mice testes. Int J Fertil Steril. 2016;10:79. doi: 10.22074/ijfs.2016.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matz EL, Hsieh MH. Review of advances in uroprotective agents for cyclophosphamide-and ifosfamide-induced hemorrhagic cystitis. Urology. 2017;100:16–9. doi: 10.1016/j.urology.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Ghobadi E, Moloudizargari M, Asghari MH, Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Exp Opin Drug Metab Toxicol. 2017 doi: 10.1080/17425255.2017.1277205. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Pagliari F, Mandoli C, Forte G, Magnani E, Pagliari S, Nardone G, et al. Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano. 2012;6:3767–75. doi: 10.1021/nn2048069. [DOI] [PubMed] [Google Scholar]
- 27.Saikia H, Hazarika KK, Chutia B, Choudhury B, Bharali P. A Simple Chemical Route toward High Surface Area CeO2 Nanoparticles Displaying Remarkable Radical Scavenging Activity. ChemistrySelect. 2017;2:3369–75. [Google Scholar]
- 28.Mittal S, Pandey AK. Cerium oxide nanoparticles induced toxicity in human lung cells: Role of ROS mediated DNA damage and apoptosis. BioMed Res Int 2014. 2014 doi: 10.1155/2014/891934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowding JM, Das S, Kumar A, Dosani T, McCormack R, Gupta A, et al. Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials. ACS Nano. 2013;7:4855–68. doi: 10.1021/nn305872d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vernet P, Aitken R, Drevet J. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004;216:31–9. doi: 10.1016/j.mce.2003.10.069. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A, Makker K, Sharma R. Review article: Clinical relevance of oxidative stress in male factor infertility: An update. Am J Reprod Immunol. 2008;59:2–11. doi: 10.1111/j.1600-0897.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 32.Cao Y, Wang X, Li S, Wang H, Yu L, Wang P. The Effects of l-Carnitine Against Cyclophosphamide-Induced Injuries in Mouse Testis. Basic Clin Pharmacol Toxicol. 2017;120:152–8. doi: 10.1111/bcpt.12679. [DOI] [PubMed] [Google Scholar]
- 33.Quita SM. Evaluation of lemon fruit extract as an antioxidant agent against histopathological changes induced by cyclophosphamide in the testes of albino mice. Electron Phys. 2016;8:1824. doi: 10.19082/1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falchi L, Bogliolo L, Galleri G, Ariu F, Zedda MT, Pinna A, et al. Cerium dioxide nanoparticles did not alter the functional and morphologic characteristics of ram sperm during short-term exposure. Theriogenology. 2016;85:1274–81. e3. doi: 10.1016/j.theriogenology.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Gupta RS, Kim J, Gomes C, Oh S, Park J, Im W-B, et al. Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol Cell Endocrinol. 2004;221:57–66. doi: 10.1016/j.mce.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh D, Das U, Ghosh S, Mallick M, Debnath J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: A correlative study with testicular oxidative stress. Drug Chem Toxicol. 2002;25:281–92. doi: 10.1081/dct-120005891. [DOI] [PubMed] [Google Scholar]
- 37.El Tawab AMA, Shahin NN, AbdelMohsen MM. Protective effect of Satureja montana extract on cyclophosphamide-induced testicular injury in rats. Chem Biol Interact. 2014;224:196–205. doi: 10.1016/j.cbi.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Howell SJ, Radford JA, Ryder WDJ, Shalet SM. Testicular function after cytotoxic chemotherapy: Evidence of Leydig cell insufficiency. J Clin Oncol. 1999;17:1493. doi: 10.1200/JCO.1999.17.5.1493. [DOI] [PubMed] [Google Scholar]
- 39.Arenas MI, Lobo MV, Caso E, Huerta L, Paniagua R, Martín-Hidalgo MA. Normal and pathological human testes express hormone-sensitive lipase and the lipid receptors CLA-1/SR-BI and CD36. Hum Pathol. 2004;35:34–42. doi: 10.1016/j.humpath.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Moore RG, Vorsa N, Singh RK. Purified cranberry proanthocyanidines (PAC-1A) cause pro-apoptotic signaling, ROS generation, cyclophosphamide retention and cytotoxicity in high-risk neuroblastoma cells. Int J Oncol. 2012;40:99–108. doi: 10.3892/ijo.2011.1225. [DOI] [PubMed] [Google Scholar]
- 41.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, et al. Dietary nickel chloride induces oxidative stress, apoptosis and alters Bax/Bcl-2 and caspase-3 mRNA expression in the cecal tonsil of broilers. Food Chem Toxicol. 2014;63:18–29. doi: 10.1016/j.fct.2013.10.033. [DOI] [PubMed] [Google Scholar]