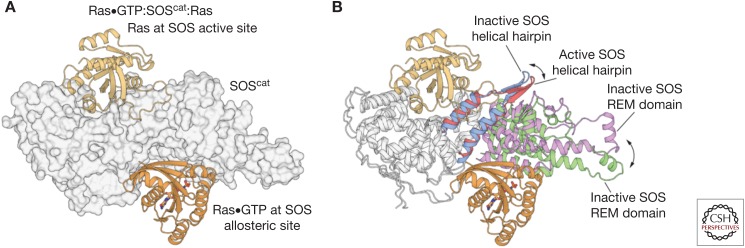
Figure 3.
The activation of Son-of-Sevenless (SOS) by Ras•GTP (guanosine triphosphate). (A) The structure of the 2:1 Ras:SOScat complex (Protein Data Bank [PDB] code: 1NVV) revealed that nucleotide-free Ras was bound to the active site of the Cdc25 domain, and that a second Ras•GTP molecule is bound to a distal allosteric site, wedged between the Ras exchanger motif (REM) and Cdc25 domains (B) Comparison of the Ras-free structure of SOScat (PDB code: 2II0) to the 2:1 Ras:SOScat complex shows that the helical hairpin is tilted toward the active site of SOS in the Ras-free state, thereby constricting the site where SOS engages Switch II of nucleotide-free Ras. Allosteric binding of Ras•GTP to SOS leads to the rotation and opening of the helical hairpin, accompanied by the outward rotation of the REM domain, which frees the catalytic site to bind Ras.