Abstract
Background:
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) can cure leukaemia. However, long term complications of post transplantation interfere with the patients’ full recovery. The objective of this review was to identify the various long term complications and to assess their individual prevalences.
Methods:
Electronic databases including PubMed, Google Scholar and Cochrane were searched for years 2004–2017. The keywords used were leukaemia, allogenic stem cell transplantation, prevalence, side effects, long term, delayed, adverse effects, complications and outcome.
Results:
A total of ten articles were included for analysis. There were 5 prospective studies, 3 retrospective studies and 2 cross sectional studies. A total of 40,069 patients, (20,189 males and 17,191 females) participated in these 10 studies. The gender of 2689 patients were not disclosed. Most common late complications and prevalence were chronic graft versus host disease (43% at 5 years post HSCT), secondary tumor (21% at 20 years post HSCT), hypothyroidism (11% at 15 years), bronchiolitis obliterans (9.7% at 122 days), cardiovascular disease (7.5% at 15 years) and avascular necrosis (5.4% at 10 years). The prevalence of azoospermia was 71.1% and depression, 18%. For the latter two conditions no time limit was available. Follow up duration ranged from 2 years till 30 years post HSCT.
Conclusion:
While allogenic stem cell transplantation is an effective cure for leukaemia, the procedure is associated with complications that can have their onset many years after the procedure.
Keywords: Allogenic stem cells, Chronic graft, Late complications, Leukaemia
Introduction
About 50,000 haematopoietic stem cells transplantations (HSCT) are performed in the world every year and the number of patients that will undergo this procedure is expected to rise. Allogenic haematopoietic stem cell transplantation is becoming more and more common with each passing day [1, 2].
This type of transplantation is a rather astonishingly effective remedy available for both cancerous and non-cancerous diseases affecting the bone marrow [3]. This procedure can be performed for both non-cancerous conditions such as severe aplastic anemia as well as cancerous conditions such as leukaemia, myelomas and lymphomas, both Hodgkins and Non Hodgkins.
Patients have been followed for up to 30 years post transplantation procedure and have actually been found to be cured of leukaemia [3]. Improvements in the treatment of the post-transplant patient has led to better survival rates after this procedure. Although recipients of allogenic HSCT enjoy remission and control of the leukaemia, being either acute myeloid leukaemia (AML), chronic myeloid leukaemia (CML), acute lymphocytic leukaemia (ALL) or chronic lymphocytic leukaemia (CLL), it may not be associated with the return to healthy baseline levels of pre-disease state [3]. The complications of the procedure experienced by survivors is definitely substantial and impacts their health and function. Escaping death because of the disease is no longer a concern but just the beginning of a whole host of possible problems [3]. For resumption of health, physical and physiological normality should be the end point that the clinician should aim for.
Nivision et al. [4] from Australia elicited that the patient’s survival was found to be 99.5% of the normal population 10 years after HSCT. It has been highlighted that those living 5 years after allogenic HSCT without any recurrence had a high chance of surviving a further 15 years although death rate remained higher than the rest of the population [4]. Current papers state that death post HSCT is due to other malignancies, recurrent disease attributed to immunocompromised health, infections such as toxoplasmosis, chronic GVHD, lung, heart and vessel problems [5]. Infections frequently occur in the acute post-transplant period due to not just bacteria, but viruses and fungi as well.
Allo-HSCT patients had twice the chance than their immediate relatives to develop a chronic health condition and if they had cGVHD, they were 4.7 times more likely to develop a condition that could lead to death [5].
Information regarding the complications of HSCT is heterogenous. Information needs to be integrated so it can be transplanted into practical care for the patient. The clinician needs to understand the complications to initiate appropriate and a precise management pertaining to the care of the patient. The objectives of this review paper are to identify and understand the complications pursuant to allogenic stem cell therapy in leukemia patients, mostly focusing on adults, to assess the prevalence of these side effects.
The term long-term refers to 2 years and more post HSCT. Previous studies have described complications arising 2 years post transplantation as late onset or delayed [3].
Chronic side effects focused on in this structured literature review include graft versus host disease (GVHD), immune system disruption, secondary cancers [3]. Data on other side effects were also collected in this review. Post-transplant allogenic patients usually enjoy fair health. However, this procedure may be associated with numerous complications for some patients due to side effects that occur after 2 years. Data about the complications in the literature is varied and conflicting, hence the clinician needs to identify and integrate this information. The present study embarked to answer the following questions:
What the side effects associated with allogenic stem cells in the treatment of leukaemia are and how prevalent these side effects are. This study will be beneficial to both the doctor and the patient. The results from this study might be helpful for doctors to implement appropriate strategies to either prevent or manage the side effects of allogenic HSCT. On the other hand, outcome of this study might be useful as educational materials for patients to be more aware of the complications of HSCT.
Materials and methods
The type of study was as a structured literature review. The inclusion criteria included patients who had allogenic transplantation performed as treatment for leukaemia. More than half the sample must have primary disease as leukaemia. It must also be either a clinical trial (case control, cohort, randomized control trial) involving delayed adverse effects regarding allogenic stem cell transplantation. The literature had to be in English and involve only human subjects. The papers should have been published between 2004 and 2017. Full text must be available.
The exclusion criteria were allogenic stem cell transplantations for non-malignant conditions as the primary disease and less than 2 years follow up post-transplant. A preliminary search using electronic databases and articles were searched on online databases using the keywords separately to ensure as many studies as possible were assessed. Relevant papers on the reference list of the viewed papers and other papers citing the viewed papers were also searched and reviewed. Refined search and Literature Selection Abstracts of the articles with titles relating to the study topics were viewed and relevant articles were downloaded for references. Literature selections was done based on inclusion and exclusion criteria. The keywords were leukaemia, allogenic stem cell transplantation, prevalence, side effects, long term, delayed, adverse effects, complications and outcome. The journal search was conducted at Pubmed, Cochrane and Google Scholar. Abstracts of the articles retrieved from the database search were reviewed carefully. Those articles that were clearly not eligible for inclusion criteria were left out. The selected list was discussed with the supervisor and conflicts were resolved after much discussion.
Results
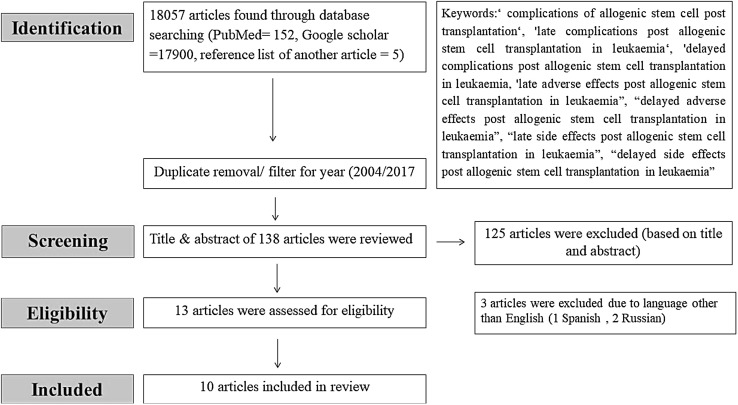
Using different keywords, a total of 18,057 articles were retrieved. After reviewing the titles and abstracts, only 10 articles were relevance to the objectives of this review (Fig. 1). The full details of each selected article are summarized in Tables 1 and 2.
Fig. 1.
Flow diagram of articles selection process
Table 1.
Summary of articles on the different types of late complications of allogenic hematopoietic stem cell transplantation
| Type of transplant | Source of stem cells | Donor characteristics | Follow up duration | Study design | No. of patient with leukemia/total (n) | Author/year |
|---|---|---|---|---|---|---|
| 1. Allogenic | BM = 5 PBSC = 269 |
Related = 118 MUD = 156 |
3 years | Prospective | AML = 274 n = 274 |
Boglarka et al. [6] |
| 2. Allogenic | N/A | Related = 2645 Mismatched related = 169 Unrelated = 494 Unknown = 29 |
25 years | Prospective | ALL = 1364 CML = 1060 n = 3337 |
Friedman et al. [7] |
| 3. Allogenic | BM = 52 PBSC = 92 |
Related = 95 MUD = 49 |
2 years | Cross sectional | AML = 47 CML = 28 ALL = 18 n = 144 |
Yoshihara et al. [8] |
| 4. Allogenic and autologous | N/A | N/A | 9 years | Retrospective | AML-79 CML = 75 ALL = 51 n = 265 |
Tichelli et al. [9] |
| 5. Allogenic and autologous | N/A | Allogenic Related = 627 Unrelated = 20 |
8.2 years | Retrospective | AML-335 CML = 230 ALL = 178 n = 1346 |
Campbell et al. [10] |
| 6. Allogenic | BM = 28 PBSC = 11 |
Matched related = 32 MUD = 7 Mismatched related = 0 |
9 years | Cross sectional | AML-17 CML = 11 ALL = 4 n = 39 |
Rovo et al. [11] |
| 7. Allogenic | BM = All patients | Matched related = 22,661 | 5 years | Prospective | AML-7461 CML = 7594 ALL = 5516 n = 28,874 |
Rizzo et al. [2] |
| 8. Allogenic | N/A | Matched related = 102 | 10 years | Prospective | CML = 102 n = 102 |
Robin et al. [12] |
| 9. Allogenic | BM 1319 PBSC 1096 |
Related Unrelated |
30 years | Prospective | AML-852 CML = 498 ALL = 299 CMML = 17 n = 2415 |
Michelis et al. [13] |
| 10. Allogenic | PBSC 1752 BM1337 Both PBSC& BM 29 Cord Blood 43 Unknown = 112 |
Syngenic 43 Matched related 2160 Mismatched related 159 Matched unrelated donor 808 Mismatched unrelated donor 103 |
2.8 years | Retrospective | AML-1110 CML = 636 ALL = 536 Other leukaemias = 43 n = 3273 |
Vajdic et al. [14] |
BM bone marrow, PBSC peripheral blood stem cell, MUD matched unrelated donor
Table 2.
Specification of HSCT treatment
| Age at HSCT | Type of complication and prevalence | Type of conditioning | Time of onset after HSCT | Characteristicsof affected patients | Reference |
|---|---|---|---|---|---|
| Mean age 60 years (range 5–74 years) | cGVHD 5 years post HSCT 43% |
RIC TBI 2Gy-28 TBI 2 Gy and FLU-246 |
Acute GVHD developed at a median of 42 days after HSCT | Had non T cell depleted BM Female donors PBSC mobilized SC History of aGvHD |
Boglarka et al. [6] |
| Mean age 28.1 years | Breast cancer 1.56% | TBI-1070 noTBI-2162 Unknown-1050 |
12.5 years (5.7–24.8 years) | Friedman et al. [7] | |
| Older than 55 years | Bronchiolitis obliterans 9.7% | MA = 96 RIC = 48 |
Median time from HSCT to onset of symptoms was 122 days | Symptoms of all 14 patients were cough, dyspnoea and wheezing | Yoshihara et al. [8] |
| Median age = 39 years | CVS problems 7.5% at 15 years post HSCT 22% at 25 years post HSCT |
TBI = 229/265 | N/A | More risk factors predisposed to more arterial events Older age at HSCT Undergoing allogenic HSCT Myeloid neoplasm as primary disease |
Tichelli et al. [9] |
| Median age 34 years (range 7 month to 69 years) | 3.7% developed avascular necrosis of bone (AVN) at post HSCT 5 years and 5.4% at post HSCT 10 years After allogenic matched related donor 5.57% developed AVN |
TBI | N/A | Tend to be male Undergoing allogenic HSCT was a risk factor Exposure to the specific chemotherapeutic factors were not risk factors for AVN |
Campbell et al. [10] |
| Median age 25 years | Azoospermia Oligospermia 71.8% |
TBI = 33 RIC = 3 | NA | Presence of cGVHD Presence of spermatogenesis was depicted by longer interval since HSCT |
Rovo et al. [11] |
| Median age 27 years | SCC Sarcoma Melanoma Breast cancer 0.65% developed solid tumors |
NO TBI | N/A | Occur more in males Twice as common compared to general population Young age at exposure predicts Non SCC occurrence Presence of cGVHD increased risk of tumor |
Rizzo et al. [2] |
| Median age 37 years | Second cancer 21% at 20 years Cataracts 25% at 15 years Hypothyroidism 11% at 15 year Depression 18% Bone and joints 6.86% had late malignancies |
TBI = 37 | Cancers occur 106 months after HSCT. Cataracts occur 31 months after HSCT. Depression occurred more in those with chronic GVHD Osteonecrosis occurred more in female < 40 years. |
No risk factors were identified | Robin et al. [12] |
| Median age 43 years | Secondary malignancies 8.7% (209/2415) 58/209 developed non metastatic squamous cell carcinoma and basal cell carcinoma |
TBI = 577 TBI 200–500 = 1240 TBI > 500 = 598 |
Median time of onset was 80 months | Older age at HSCT was a risk factor for secondary malignancies TBI dose, graft source and donor type did not have any significant impact on the development of secondary malignancies |
Michelis et al. [13] |
| Median age 40.4 years | Secondary malignancies 79 second primary Cancers in 76 out of 3273 patients. |
Limited field irradiation 2 Low dose TBI 762 High dose TBI 252 Unknown dose TBI 477 |
3.54 years after HSCT The risk of Melanoma was increased first year post HSCT Lip and tongue cancer elevated all time beyond the first year Soft tissue sarcomas were observed within 4 years Risk of brain, thyroid cancer and non-Hodgkins lymphoma was increased in the first year after HSCT |
Risk of cancer was elevated in those who developed c GVHD Risk was not related to the source of the stem cells or whether radiation based conditioning was received |
Vajdic et al. [14] |
CVS cardiovascular system, TBI total body radiation, MA myeloablation, RIC reduced intensity conditioning, Gy gray, FLU fludarabine, SCC squamous cell carcinoma
Discussion
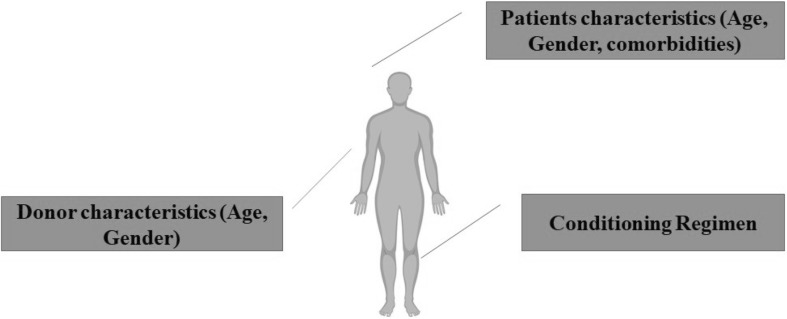
A variety of complications have been reported in the 10 selected studies. These were included; GVHD, bronchiolitis obliterans avascular necrosis, cardiovascular disorders, azoospermia, secondary tumors, depression, and hypothyroidism. Table 3 and Fig. 2 schematically summarize the different steps that may be considered as risk factors for long term complications of allo-HSCT.
Table 3.
Risk factors for common late complications of allo-HSCT
| Late complications | Risk factors |
|---|---|
| cGVHD | (a) Donor characteristics Matched sibling donor is better than matched unrelated donor and is in turn better than haplo-identical unmatched donor [15] Gender Males make better donors than previously pregnant females [16] (b) Graft source/characteristics Umbilical cord blood is associated with less risk due to immunological immaturity of T cells. [17, 18] PBSCT associated with higher risk than BMT [19] (c) Stem cells manipulation with T cell depleted better than T cell replete [20] (e) Cytomegalovirus status [21] |
| Secondary malignancies | (a) Prior therapy: chemotherapy/radiotherapy [22] (b) Conditioning regimen: myelo ablative/reduced intensity conditioning [22] |
| Organ toxicity | (a) Prior therapy: chemotherapy/radiotherapy [22]. (b) Conditioning regimen: myeloablative/reduced intensity conditioning [22] (c) Post-transplant immunosuppression with busulphan and methotrexate are risk factors for the development of BO [23] |
| Infertility | (a) Total body irradiation predisposes to infertility [22] |
Fig. 2.
Different elements that may be considered as risk factors for long-term side effects after allo-HSCT
One of the important principles of a conditioning regimen is that reduced intensity conditioning has a better outcome compared to total body irradiation and is safer for those patients who are older and medically unwell [7, 8]. Using reduced intensity conditioning, where both low doses of chemotherapy and irradiation are used to destroy the host bone marrow, full destruction does not occur, instead a state of mixed chimerism exists whereby the host and donor cells co-exist. The benefit is due to the graft versus tumor effect [7, 8].
Graft versus host disease
Chronic graft-versus-host disease (cGVHD) is the most common late complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT) as stated by Hingman and Vogelsang [24]. In another study, a prevalence of 43% cGVHD after allo-HSCT was reported [6]. Our value seems to be lower than other published studies. A study by Mielcarek et al. [25] reported a much higher prevalence of 73%. Skert et al. [26] noted that 75% of patients developed cGVHD after allo-HSCT. Both acute and chronic GVHD are more common after PBSCT than BMT, and this may also be associated with lower rates of malignant relapse [27]. The magnitude of the transfused T cell load may explain the differences in cGVHD risk [27].
Secondary tumors
The prevalence of secondary tumors was 21% at 20 years and composed mainly of squamous cell cancer, sarcoma, melanoma and breast cancer. A very early study by Kolb et al. [28] that included more than 1000 patients transplanted before December 1985, showed that the incidence of secondary malignancies ranged from 3.5% at 10 years and 12.8% at 15 years, and this is 3.8-fold higher than that of an age-matched control population. This figure was recently updated and it showed that the incidence of secondary malignancies continued to increase with longer follow-up time compared with age-matched control population [29]. This fact is concordant with the findings in our literature review.
Bronchiolitis obliterans (BO)
BO occurred in 9.7% of the patients in the study by Boglarka et al. [6]. However, the prevalence of BO varied within a wide range as low as 2–3% in a study by Chien et al. [30], 5.5% in a study by Brandon et al. [31], and these values increase to 14% when BO is associated with cGVHD [31]. According to Brandon et al. [31] the presence of cGVHD is said to increase the risk tenfold. In the study by Brandon et al. the median time from procedure to attaining the spirometric definition for bronchiolitis obliterans syndrome was 439 days (range 274–1690). While most previously identified risk factors were not significantly associated with bronchiolitis obliterans, not being Caucasian (p = 0.014), lower circulating IgG levels (p = 0.010) and the presence of cGVHD (p < 0.001) were associated with an increase in risk. Bronchiolitis obliterans syndrome conferred a 1.6 fold increase in risk for mortality after diagnosis [31]. Spirometric monitoring of high-risk patients with cGVHD might allow early diagnosis and treatment for this frequently fatal disease.
Avascular necrosis (AVN)
In the study by Campbell et al. [10] the prevalence of AVN was 3.7%, 5 years post HSCT and 5.4%, 10 years post HSCT. The incidence according to Weisman et al. can range from 6.3% at initial evaluation and 11.8% for long-term survivors [32]. The mean time from procedure to detection of the condition was 13 months [32]. According to Robin et al. [2] this condition was seen more in younger women less than 40 years of age.
Cardiovascular side effects
The prevalence of cardiovascular side effects was 7.5% at 15 years post HSCT and was 22% at 25 years post HSCT. Allogeneic recipients with hypertension were found to have a 2.5-fold increased risk of a cardiovascular event, while those with diabetes had a 2.3-fold increased risk [9].
Azoospermia and oligospermia
The above complication occurred at a prevalence of 72% in the study by Rovo et al. [11].
Depression
Was associated with the concurrent presence of cGVHD. The prevalence of this condition was 18%.
Conclusion
As it could be seen allogenic HSCT is a good therapy for leukaemia but it has multiple associated late complications that debilitate the patient. The therapy will be more effective if these side effects are better managed. Most common late complications and prevalence were chronic graft versus host disease 43% at 5 years post HSCT, secondary tumor 21% at 20 years post HSCT, hypothyroidism 11% at 15 years, bronchiolitis obliterans 9.7% at 122 days, cardiovascular disease 7.5% at 15 years and avascular necrosis 5.4% at 10 years. Azoospermia was 71.1%. Depression was 18%. For the latter 2 condition no time limit was available. Follow up duration ranged from 2 years post HSCT to 30 years. It was very evident that cGVHD occurred concurrently with a variety of conditions such as bronchiolitis obliterans, atherosclerosis and avascular necrosis. Based on this literature review, it could be presumed that controlling the occurrence of cGVHD through using more matched and donor related transplantations will improve the outcome of allogenic transplantation. This issue must definitely be addressed as more individuals would opt for this procedure. This review study concludes that while allogenic HSCT is an effective cure for leukaemia, the procedure is associated with side effects that can have their onset years after the procedure.
Limitations
Some of the limitations are that there were no randomized control trials among our selected studies.
There was only one paper for most of the various types of complications except for secondary tumors where there were five. The sample of patients included in the studies suffered from all kinds of blood disorders such as aplastic anemia and myelofibrosis, not just leukaemia. Concerning leukaemia per say there are four kinds of leukaemia. These are a heterogeneous group of disorders. Most of the studies did not differentiate between the various types of leukaemias nor did they differentiate between HSCT given for malignant and benign conditions. As for the ethnicity, of the sample population they were mostly Caucasian and the findings cannot be generalized to other ethnicities.
Future recommendations
Firstly, similar studies should be carried out in the East as Asians are genetically different from Caucasians. Secondly, more studies should be carried out to ascertain each of the above complications in greater details. Thirdly, there should be more randomized control trials, which are the gold standard in research. Furthermore, different types of leukaemia should be studied individually as they are etiologically and histologically different from each other. At present, most of the studies have clumped the patients with various diagnoses together. In addition, after attempts to decrease the co-occurrence of cGVHD, the investigation should be done to explore if the prevalence of the other complications is decreased.
Author’s contribution
Associate Professor Dr. Sharmilla Kanagasundram acquisition, interpretation and analysis, drafting article and revising it for intellectual content. Associate Professor Farhanaz Amini concept and design of study and revising it for intellectual content.
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
There are no animal experiments carried out for this article.
Contributor Information
Sharmilla Kanagasundram, Phone: +60173368111, Email: sharmilla_kanagasundram@yahoo.com.
Farhanaz Amini, Phone: +60173530706, Email: farahnaz@ucsiuniversity.edu.my.
References
- 1.Antjin JH. Clinical practice. Long-term care after hematopoietic-cell transplantation in adults. Engl J Med. 2002;347:36–42. doi: 10.1056/NEJMcp010518. [DOI] [PubMed] [Google Scholar]
- 2.Rizzo JD, Curtis RE, Socié G, Sobocinski KA, Gilbert E, Landgren O, et al. Solid cancers after allogenic heamatopoietic stem cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohty M, Apperley JF. Long-term physiological side effects after allogeneic bone marrow transplantation. Hematol Am Soc Hematol Educ Program. 2010;2010:229–36. doi: 10.1182/asheducation-2010.1.229. [DOI] [PubMed] [Google Scholar]
- 4.Nivison-Smith I, Simpson JM, Dodds AJ, Ma DD, Szer J, Bradstock KF. Relative survival of long-term hematopoietic cell transplant recipients approaches general population rates. Biol Blood Marrow Transplant. 2009;15:1320–1330. doi: 10.1016/j.bbmt.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Martin PJ, Counts GW, Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after haemopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Nonmyeloablative allogenic haematopoietic cell transplantation in patients with acute myeloid leukaemia. J Clin Oncol. 2010;28:2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman DL, Rovo A, Leisenring W, Locasciulli A, Flowers ME, Tichelli A, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111:939–944. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshihara S, Tateisha U, Ando T, Kunitah H, Suyama H, Onishi Y, et al. Lower incidence of Bronchiolitis obliterans in allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning compared with myeloablative conditioning. Bone Marrow Transplant. 2005;35:1195–2000. doi: 10.1038/sj.bmt.1704985. [DOI] [PubMed] [Google Scholar]
- 9.Tichelli A, Passweg J, Wójcik D, Rovó A, Harousseau JL, Massi T, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 10.Campbell S, Sun CL, Kurian S, Francisco L, Carter A, Kulkarni S, et al. Predictors of avascular necrosis of bone in long term survivors of haematopietic stem cells. Cancer. 2009;115:4127–4135. doi: 10.1002/cncr.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovó A, Tichelli A, Passweg JR, Heim D, Meyer-Monard S, Holzgreve W, et al. Spermatogenesis in long-term survivors after allogenic haematopoietic stem cell transplantation is associated with age, time interval since transplantations, and apparently absence of chronic GvHD. Blood. 2006;108:1100–1105. doi: 10.1182/blood-2006-01-0176. [DOI] [PubMed] [Google Scholar]
- 12.Robin M, Guardiola P, Devergie M, Yeshurun M, Shapiro S, Esperou H, et al. A 10-year median follow-up study after allogenic stem cell transplantation for chronic myeloid leukaemia in chronic phase from HLA-identical sibling donors. Leukemia. 2005;19:1613–1620. doi: 10.1038/sj.leu.2403821. [DOI] [PubMed] [Google Scholar]
- 13.Michelis FV, Kotchetkov R, Grunwald RM, Azeem A, Atenafu EG, Lipton JH, et al. Long-term incidence of secondary malignancies after allogenic stem cell transplantation: a single-center experience. Biol Blood Marrow Transplant. 2017;23:945–951. doi: 10.1016/j.bbmt.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Vajdic CM, Mayson E, Dodds AJ, O’Brien T, Wilcox L, Nivision-Smith I, et al. Second cancer risk and late mortality in adult Australians receiving allogeneic hematopoietic stem cell transplantation: a population-based cohort study. Biol Blood Marrow Transplant. 2016;22:949–956. doi: 10.1016/j.bbmt.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Zhou D, Guo D, Xu P, Chen B. Comparison of outcomes in hematological malignancies treated with haploidentical or HLA-identical sibling hematopoietic stem cell transplantation following myeloablative conditioning: a meta-analysis. PLoS One. 2018;13:e0191955. doi: 10.1371/journal.pone.0191955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar AJ, Kim S, Hemmer MT, Arora M, Spellman SR, Pidala JA, et al. Graft-versus-host disease in recipients of male unrelated donor compared with parous female sibling donor transplants. Blood Adv. 2018;2:1022–1031. doi: 10.1182/bloodadvances.2017013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SB, Madrigal JA. Immunological and functional differences between cord and peripheral blood. Bone Marrow Transplant. 1998;21 Suppl 3:S9–12. [PubMed]
- 18.Atsuta Y, Suzuki R, Nagamura-Inoue T, Taniguchi S, Takahashi S, Kai S, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto TE, Yamaguchi T, Tanaka Y, Saito A, Tajima K, Karasuno T, et al. Comparative analysis of clinical outcomes after allogeneic bone marrow transplantation versus peripheral blood stem cell transplantation from a related donor in Japanese patients. Br J Haematol. 2004;125:480–493. doi: 10.1111/j.1365-2141.2004.04943.x. [DOI] [PubMed] [Google Scholar]
- 20.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, et al. Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts. J Clin Invest. 2015;125:2677–2689. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lönnqvist B, Ringdén O, Wahren B, Gahrton G, Lundgren G. Cytomegalovirus infection associated with and preceding chronic graft-versus-host disease. Transplantation. 1984;38:465–468. doi: 10.1097/00007890-198411000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev Hematol. 2011;4:437–454. doi: 10.1586/ehm.11.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santo Tomas LH, Tomas LH, Loberiza FR, Jr, Klein JP, Layde PM, Lipchik RJ, Rizzo JD, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128:153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 24.Hingman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol. 2004;125:435–454. doi: 10.1111/j.1365-2141.2004.04945.x. [DOI] [PubMed] [Google Scholar]
- 25.Mielcarek M, Martin PJ, Leisering W, Flowers ME, Maloney DG, Sandmaier BM, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 26.Skert C, Patricia F, Sperotto A, Cerno M, Fili C, Zaja F, et al. Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica. 2006;91:258–261. [PubMed] [Google Scholar]
- 27.Cutler C, Antin JH. Peripheral blood stem cells for allogeneic transplantation: a review. Stem Cells. 2001;19:108–117. doi: 10.1634/stemcells.19-2-108. [DOI] [PubMed] [Google Scholar]
- 28.Kolb HJ, Socié G, Duell T, Van Lint MT, Tichelli A, Apperley JF, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- 29.Heilmeier B, Stowasser N, Socie G, Van Lint MT, Tichelli A, Salooja N, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation—follow up. Blood. 2008;112:453. doi: 10.1182/blood-2008-05-153585. [DOI] [PubMed] [Google Scholar]
- 30.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:S106–S114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiesmann A, Pereira P, Böhm P, Faul C, Kanz L, Einsele H. Avascular necrosis of bone following allogeneic stem cell transplantation: MR screening and therapeutic options. Bone Marrow Transplant. 1998;22:565–569. doi: 10.1038/sj.bmt.1701374. [DOI] [PubMed] [Google Scholar]