Abstract
Coronary heart disease is a major health problem in developed countries. Many studies have shown that elevated serum concentrations of total or low-density-lipoprotein cholesterol (LDL cholesterol) are high risk factors, whereas high concentrations of high-density-lipoprotein cholesterol (HDL cholesterol) or a low LDL to HDL cholesterol ratio may protect against coronary heart disease. Plant sterols and stanols derived from vegetable oils or wood pulp have been shown to lower total and LDL cholesterol levels in humans by inhibiting cholesterol absorption from the intestine. These findings may lead to new therapeutic options to treat hypercholesterolemia. In addition, phytosterols may influence cell growth and apoptosis of tumor cells. However, they can interfere with the absorption of fat soluble vitamins and carotenoids.
Keywords: Cholesterol, Cardiovascular, Cancer, Sitosterol, Campesterol, Plant stanol
1. Introduction
A close correlation between blood cholesterol levels and heart disease has been reported in humans. Deposition of cholesterol, cholesterol esters and other lipids in the artery wall, leads to narrowing and hardening of the artery, which in turn may lead to atherosclerosis and an increased risk of forming blood clots (thrombosis).
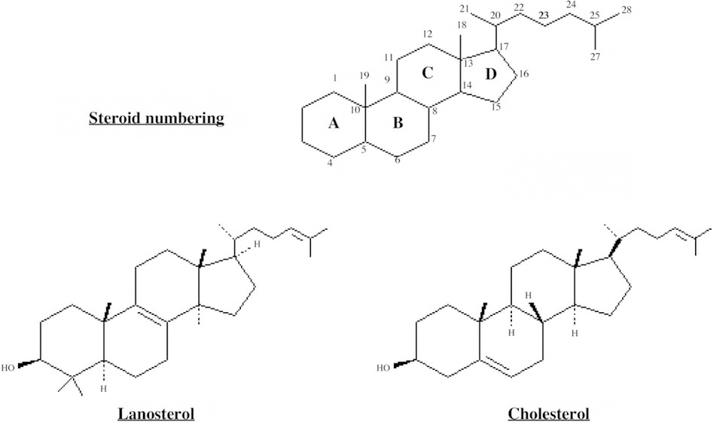
Cholesterol is found in all animal tissues where the majority serves as a structural element in cell membrane walls, whilst the remainder is transported via the blood and acts as a precursor for other steroid based molecules such as sex hormones, steroid hormones, vitamin D or bile acids. The major sterol found in mammals is the C–27 compound cholesterol (Fig. 1).
Fig. 1.
Chemical structure of cholesterol and lanosterol.
In animals the triterpenoid alcohol lanosterol is converted into cholesterol, a process requiring the loss of three methyl groups, reduction of the side-chain double bond and generation of a Δ5,6 double bond in place of the Δ8,9 double bond (Fig. 1). Cholesterol is currently available in quantity via the brain and spinal cord of cattle as a by-product of meat production, and these, form one source for medicinal steroid semi-synthesis. Large quantities are also extractable from lanolin, the fatty material coating sheep’s wool. Saponification of crude lanolin gives an alcohol fraction (lanolin alcohols or wool alcohols) containing about 34% cholesterol and 38% lanosterol/dihydrolanosterol. Wool alcohols are also used as an ointment base. Bile acid is obtained by purification from fresh ox bile taken from carcasses as a by-product of the meat trade. It is still important as a starting material for the semi-synthesis of other medicinal steroids being a cheap and readily accessible raw material.
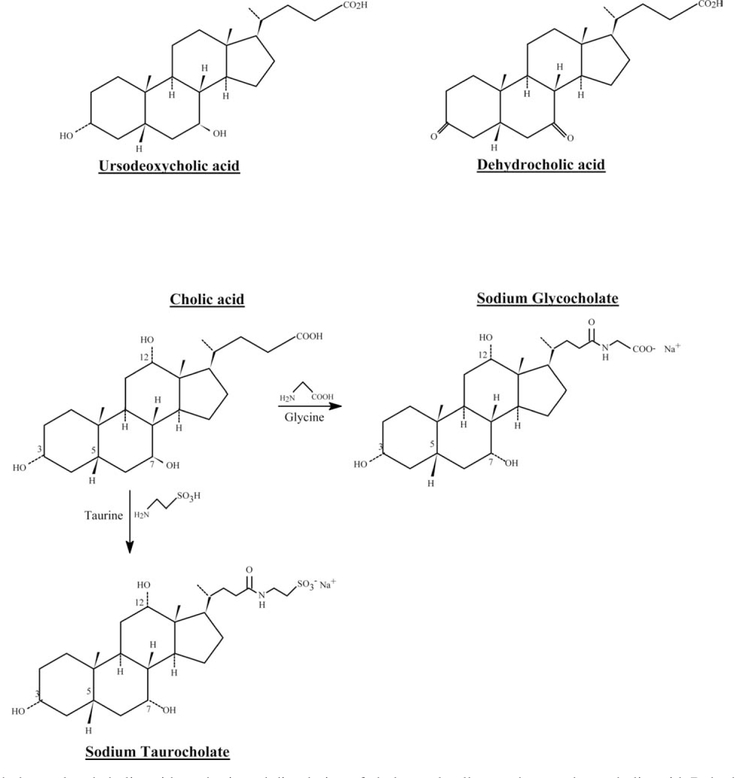
Some human gallstones are almost entirely composed of cholesterol precipitated from the bile. Chenodeoxycholic acid and ursodeoxycholic acid are used to dissolve cholesterol gallstones as an alternative to surgery. By suppressing synthesis of both cholesterol and cholic acid, they contribute to removal of biliary cholesterol and consequently a gradual dissolution of gallstones, which may have formed due to supersaturation. Partial or complete dissolution requires treatment over a period of many months and is not effective for radio-opaque gallstones, which contain appreciable levels of calcium salts. Dehydrocholic acid may be used after surgery to improve biliary drainage (Fig. 2). Anion-exchange resins such as colestyramine and cholestipol are used as cholesterol-lowering drugs to bind bile acids and prevent their reabsorption. This promotes hepatic conversion of cholesterol into bile acids, increasing breakdown of low-density-lipoprotein cholesterol (LDL cholesterol).
Fig. 2.
Suppression of cholesterol and cholic acid synthesis and dissolution of cholesterol gallstones by ursodeoxycholic acid. Dehydrocholic acid is used to improve biliary drainage.
Transport of cholesterol is facilitated by formation of lipoprotein carriers, comprising protein and phospholipid shells surrounding a core of cholesterol, in both free and esterified forms. Risk of atherosclerosis increases with increasing levels of LDL cholesterol, and is reduced with increasing levels of high-density-lipoprotein cholesterol (HDL cholesterol). Blood LDL-cholesterol levels are thus a good statistical indicators of the potential risk of a heart attack. The risks can be lessened by avoiding food rich in cholesterol. In humans dietary, cholesterol is actually a smaller contributor to LDL-cholesterol levels than is dietary saturated fat.
2. Protection from cardiovascular diseases
2.1. Cholesterol-lowering effıcacy of phytosterols
Cholesterol biosynthesis may be inhibited by drug therapy using specific inhibitors of the mevalonate pathway (lovastatin and related compounds). Blood LDL-cholesterol levels may be reduced by incorporating sterol or stanol esters into the diet which reduce the absorption of cholesterol.
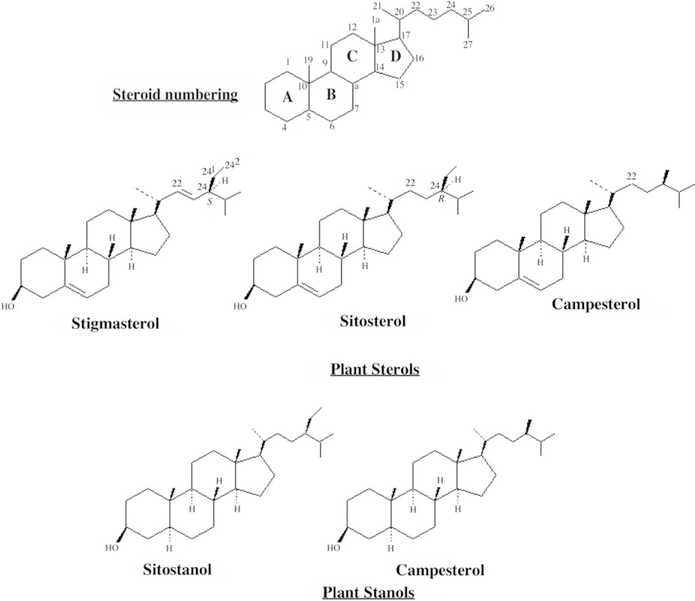
The main sterols in plants, are characterized by extra one carbon or two carbon substituents on the side-chain, attached at C–24. These substituent carbons are numbered 241 and 242. The widespread plant sterols campesterol and sitosterol are, respectively, 24–methyl and 24–ethyl analogues of cholesterol. Stigmasterol contains additional unsaturation in the side chain a trans Δ22 double bond, a feature seen in many plant sterols but never in mammalian ones (Fig. 3).
Fig. 3.
Chemical structure of phytosterols and plant stanols.
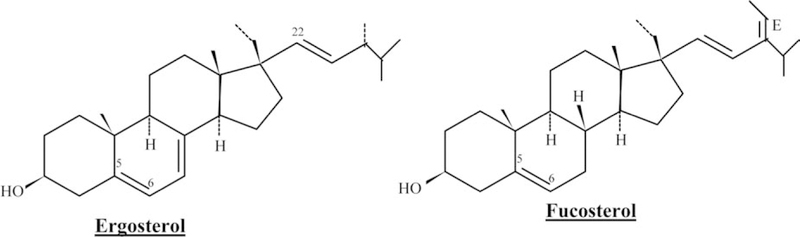
In fungi, ergosterol is the predominant sterol which has a β oriented 24–methyl as well as trans Δ22 double bond and additional Δ7 unsaturation and the most abundant sterol in brown algae is fucosterol (Fig. 4). Soybeans contain substantial amounts of sterols (about 0.2%). These include stigmasterols (about 20%), sitosterol (about 50%) that are used for the semi-synthesis of medicinal steroids and campesterol (about 20%). About 40% of the sterol content is in the free form, the remainder being combined in the form of glycosides or as esters with fatty acids.
Fig. 4.
Chemical structure of fungal sterols.
Related materials used in a similar way are plant stanol esters (Fig. 3). Stanols are obtained by hydrogenation of plant sterols and consist mainly of sitostanol (from sitosterol and stigmasterol) and campestanol (from campesterol). These are then esterified with fatty acids. The stanols are usually transesterified with rapeseed oil which is unsaturated fatty acid.
The efficacy of dietary plant sterols in reducing cholesterol levels has led to the introduction of plant sterol esters as food additives as an aid to reducing blood levels of LDL cholesterol [1].
Plant sterols are more hydrophobic than cholesterol and have greater affinity to micelles involved in fat digestion. Thus, they can displace intestinal cholesterol from the micelles, reducing intestinal cholesterol absorption [2]. This reduction may result in a compensatory increase in endogenous cholesterol synthesis [3], higher LDL-receptor expression [4], and lower circulating LDL-cholesterol concentrations [5–7].
Sterols are forming highly stable crystals in which the hydrophilic hydroxyl groups are sequestered inside the matrix and are unavailable to solubilizing fluids. As a result, plant sterols and stanols dissolve slowly in bile salt and micelles [8], are less absorbed, their biliary excretion is faster and consequently their serum levels are very low [9]. Thus, plant sterols (such as sitosterol from soybeans) or sitostanol (a 5-α-reduced metabolite of the common plant sterol, β-sitosterol) esters are usually esterified with fatty acids to produce a fat soluble product.
Plant sterols and sitostanol lower circulating cholesterol concentrations with great variability (total and LDL-cholesterol concentrations decreased by 5–15%) [10–20].
Tall oil is the fat soluble fraction of the hydrolysate obtained from trees during the pulping process. Supplementation with 22 mg per kg body wt-1/d–1 tall oil phytosterols was effective in lowering circulating LDL-cholesterol concentrations in hypercholesterolemic subjects without changing either endogenous cholesterol synthesis or phytosterols concentrations [21]. Both sitostanol and β-sitosterol esters are proposed to reduce plasma cholesterol concentrations by competitively blocking cholesterol absorption from the intestinal lumen, displacing cholesterol from bile salt micelles, increasing bile salts excretion or hindering the cholesterol esterification rate in the intestinal mucosa [9,22–25] while stimulating [26,27], inhibiting [28] or having no effect [29] on cholesterol synthesis. Moreover, phytosterol may act on hepatic acetyl-CoA carboxylase and 7-α hydroxylase enzyme activities in animals and humans [30,31].
Although, plant sterols and stanols have no adverse effects [32], they may act on fat soluble components other than cholesterol, such as vitamins and antioxidants that might be reduced as well.
Carotenoids and tocopherols are transported by lipoproteins, like cholesterol. Thus, after consumption of plant sterols or stanols, the plasma concentrations of carotenoids and tocopherols decrease, whereas plasma concentrations of retinol, 25-hydroxy-vitamin D and vitamin K were unaffected by dietary plant sterols and stanols esters [33].
Replacement of intestinal cholesterol from the micelles is not the only mechanism by which plant sterols and stanols lower LDL cholesterol. When consumed as part of a diet low in fat and cholesterol [34,35], dietary and biliary cholesterol absorption in the intestine are both suppressed. Moreover, plant sterols and stanols esters have the potential to be effective in combination with cholesterol-lowering drugs [36].
3. Protection from colon, breast and prostate cancer
Although, it was reported that phytosterols might protect from cancer development, the mechanism of protection remains unknown although different mechanisms have been proposed [37–40]. Prostatic 5α-reductase and prostatic aromatase activities were decreased in rats supplemented with phytosterols [41,42] indicating that they may suppress prostate metabolism and growth. Sitosterol has been shown to alter tumor growth in independent studies [43–46]. The in-corporation of sitosterol in HT-29 cells membrane resulted in a significant decrease in sphingomyelin and an increase in phosphatidylcholine [43]. Thus the inhibition of tumor growth could be explained by phytosterols effect on sphingomyelin cycle and increased production of ceramide, which suggest alteration of signal transduction pathways [44,45]. It has also been observed that liver cell membrane-fluidity decreased by feeding rats with sitosterol [46]. Membrane fluidity is influenced by the lipid composition and particularly by the ratio cholesterol/phospholipids in the membrane [46–51]. Thus changes in membrane fluidity may also induce alteration in signal transduction affecting cell growth and differentiation in transformed cells. Additionally, sitosterol was shown not only to inhibit growth but also to induce apoptosis in human breast cancer cells [39]. Collectively these studies suggest that phytosterol supplementation may alter or delay tumor progression. As mentioned in the previous section, plant sterols may act on other fat soluble component, such as vitamins and antioxidants.
References
- [1].Ling WH, Jones PJ. Mini-review of dietary phytosterols: a review of metabolism, benefits and side effects. Life Sci 1995;57:195–206. [DOI] [PubMed] [Google Scholar]
- [2].Plat J, Mensink RP. Effects of plant sterols and stanols on lipid metabolism and cardiovascular risk. Nutr Metab Cardiovasc Dis 2001;11:31–40. [PubMed] [Google Scholar]
- [3].Miettinen TA, Gylling H. Regulation of cholesterol metabolism by dietary plant sterols. Curr Opin Lipidol 1999;10:9–14. [DOI] [PubMed] [Google Scholar]
- [4].Plat J, Mensink RP. Effect of plant stanol esters on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase mRNA expression on mononuclear blood cells of healthy men and women. FASEB J 2002;16:258–60. [DOI] [PubMed] [Google Scholar]
- [5].Hallikainen MA, Uusitupa MI. Effects of two low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am J Clin Nutr 1999;69:403–10. [DOI] [PubMed] [Google Scholar]
- [6].Gylling H, Miettinen TA. Cholesterol reduction by different plant stanol mixtures and wit variable fat intake. Metabolism 1999;48:575–80. [DOI] [PubMed] [Google Scholar]
- [7].Plat J, Mensink RP. Vegetable oil based versus wood based stanol ester mixtures: effect on serum lipids and hemostatic factors in non-hypercholeterolemic subjects. Atherosclerosis 2000;148:101–12. [DOI] [PubMed] [Google Scholar]
- [8].Armstrong MJ, Carey MC. Thermodynamic and molecular determinants of sterol solubilities in bile salt micelles. J Lipid Res 1987;28: 1144–55. [PubMed] [Google Scholar]
- [9].Heinemann T, Axtmann G, Von Bergmann K. Comparison of intestinal absorption of cholesterol with different plant sterols in man. Eur J Clin Invest 1993;23:827–31. [DOI] [PubMed] [Google Scholar]
- [10].Ostlund RE Jr, Spilburg CA, Stenson WF. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am J Clin Nutr 1999;70:826–31. [DOI] [PubMed] [Google Scholar]
- [11].Vanhanen HT, Miettinen TA. Effects of unsaturated and saturated dietary plant sterols on their serum contents. Clin Chim Acta 1992; 205:97–107. [DOI] [PubMed] [Google Scholar]
- [12].Heinemann T, Kullak-Ublick GA, Pietruck B, von Bergmann K. Mechanisms of action of plant sterols on inhibition of cholesterol absorption. Comparison of sitosterol and sitostanol. Eur J Clin Pharmacol 1991;40:S59–63. [PubMed] [Google Scholar]
- [13].Becker M, Staab D, von Bergmann K. Treatment of severe familial hypercholesterolemia in childhood with sitosterol and sitostanol. J Pediatr 1993;122:292–6. [DOI] [PubMed] [Google Scholar]
- [14].Miettinen TA, Puska P, Gylling H, Vanhanen HT, Vartiainen E. Reduction of serum cholesterol with sitostanolester margarine in a mildly hypercholesterolemic population. N Engl J Med 1995;333:1308–12. [DOI] [PubMed] [Google Scholar]
- [15].Gylling H, Siimes MA, Miettinen TA. Sitostanol ester margarine in dietary treatment of children with familial hypercholesterolemia. J Lip Res 1995;36:1807–12. [PubMed] [Google Scholar]
- [16].Vanhanen HT, Kajander L, Lehtovirta H, Miettinen TA. Serum levels, absorption efficiency, faecal elimination and synthesis of cholesterol during increasing doses of dietary sitostanol esters in hypercholesterolemic subjects. Clin Sci 1994;87:61–7. [DOI] [PubMed] [Google Scholar]
- [17].Gylling H, Miettinen TA. Serum cholesterol and lipoprotein metabolism in hypercholesterolemic NIDDM patients before and during sitostanol ester-margarine treatment. Diabetologia 1994;37:773–80. [DOI] [PubMed] [Google Scholar]
- [18].Miettinen TA, Vanhanen HT. Dietary sitostanol related to absorption phenotypes. Atherosclerosis 1994;105:217–26. [DOI] [PubMed] [Google Scholar]
- [19].Weststrate JA, Meijer GW. Plant sterol-enriched margarines and reduction of plasma total and LDL-cholesterol concentrations in normocholesterolemic and mildly hypercholesterolemic subjects. Eur J Clin Nutr 1998;52:334–43. [DOI] [PubMed] [Google Scholar]
- [20].Jones PJH, Howell T, MacDougall DE, Feng JY, Parsons W. Short-term administration of tall oil phytosterols improves plasma lipid profiles in subjects with different cholesterol levels. Metabolism 1998;47:751–6. [DOI] [PubMed] [Google Scholar]
- [21].Jones PJH, Ntanios FY, Raeini-Srjaz M, Vanstone CA. Cholesterol-lowering efficacy of a sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am J Clin Nutr 1999;69:1144–50. [DOI] [PubMed] [Google Scholar]
- [22].Child P, Kuksis A. Investigation of the role of micellar phospholipid in the preferential uptake of cholesterol over sitosterol by dispersed rat jejunal villus cells. Biochem Cell Biol 1986;64:847–53. [DOI] [PubMed] [Google Scholar]
- [23].Salen G, Ahrens EH Jr, Grundy SM. Metabolism of β-sitosterol in man. J Clin Invest 1970;49:952–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Child P, Kuksis A. Critical role of ring structure in the differential uptake of cholesterol and plant sterols by membrane preparations in vitro. J Lipid Res 1983;24:1196–209. [PubMed] [Google Scholar]
- [25].Ikeda I, Sugano M. Some aspects of mechanism inhibition of cholesterol absorption by β-sitosterol. Biochim Biophys Acta 1983;732: 651–8. [DOI] [PubMed] [Google Scholar]
- [26].Grundy SM, Ahrens EH Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res 1969;10: 304–15. [PubMed] [Google Scholar]
- [27].Konlande JE, Fisher H. Evidence for a nonabsorptive antihypercholesterolemic action of phytosterols in chicken. J Nutr 1969;98:435–42. [DOI] [PubMed] [Google Scholar]
- [28].Kakis G, Kuksis A. Effect of intravenous infusion of intralipid, cholesterol, and plant sterols on hepatic cholesterogenesis. Can J Biochem Cell Biol 1984;62:1–10. [DOI] [PubMed] [Google Scholar]
- [29].Subbiah MT, Kuksis A. Differences in metabolism of cholesterol and sitosterol following intravenous injection in rats. Biochim Biophys Acta 1973;306:95–105. [DOI] [PubMed] [Google Scholar]
- [30].Laraki L, Pelletier X, Mourot J, Debry G. Effects of dietary phytosterols on liver lipids and lipid metabolism enzymes. Ann Nutr Metab 1993;37:129–33. [DOI] [PubMed] [Google Scholar]
- [31].Shefer S, Salen G, Bullock J, et al. The effect of increased hepatic sitosterol on the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and cholesterol 7 α-hydroxylase in the rat and sitoster-olemic homozygotes. Hepatology 1994;20:213–9. [DOI] [PubMed] [Google Scholar]
- [32].Plat J, Mensink RP. Effects of plant sterols and stanols on lipid metabolism and cardiovascular risk. Nutr Metab Cardiovasc Dis 2001;11:31–40. [PubMed] [Google Scholar]
- [33].Plat J, Kerckhoffs DA, Mensink RP. Therapeutic potential of plant sterols and stanols. Curr Opin Lipidol 2000;11:571–6. [DOI] [PubMed] [Google Scholar]
- [34].Hallikainen MA, Uusitupa MI. Effects of two low-fat stanol estercontaining margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am J Clin Nutr 1999;69:403–10. [DOI] [PubMed] [Google Scholar]
- [35].Jones PJ, Ntanios FY, Raeini-Sarjaz M, Vanstone CA. Cholesterol-lowering efficacy of sitostanol-containing phytosterol mixture with a prudent diet in hyperlipidemic men. Am J Clin Nutr 1999;69:1144–50. [DOI] [PubMed] [Google Scholar]
- [36].Blair SN, Capuzzi DM, Gottlieb SO, Nguyen T, Morgan JM, Cater NB. Incremental reduction of serum total cholesterol and low-density-lipoprotein cholesterol with the addition of plant stanol ester-containing spread to statin therapy. Am J Cardiol 2000;86:46–52. [DOI] [PubMed] [Google Scholar]
- [37].Raicht RF, Cohen BI, Fazzini E, Sarwal A, Takahashi M. Protective effect of plant sterols against chemically-induced colon tumor in rats. J Cancer Res 1980;40:403–5. [PubMed] [Google Scholar]
- [38].Awad AB, Gan Y, Fink CS. Effect of beta-sitosterol, a plant sterol on growth, protein phosphatase 2A and phospholipids D in NcaP Cells. Nutr Cancer 2000;36:74–8. [DOI] [PubMed] [Google Scholar]
- [39].Awad AB, Downie D, Fink CS. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int J Mol Med 2000;5:541–5. [DOI] [PubMed] [Google Scholar]
- [40].Rao AV, Janezic SA. The role of dietary phytosterols in colon carcino-genesis. Nutr Cancer 1992;18:43–52. [DOI] [PubMed] [Google Scholar]
- [41].Mettlin C Clinical oncology update: prostate cancer. Recent developments in the epidemiology of prostate cancer. Eur J Cancer 1997;33: 340–7. [DOI] [PubMed] [Google Scholar]
- [42].Awad AB, Hartati MS, Fink CS. Phytosterol feeding induces alteration in testosterone metabolism in rat tissues. J Nutr Biochem 1998; 9:712–7. [Google Scholar]
- [43].Awad AB, Chen YC, Fink CS, Hennessey T. Beta-sitosterols inhibits HT-29 human colon cancer cell growth and alters membrane lipids. Anticancer Res 1996;16:2797–804. [PubMed] [Google Scholar]
- [44].Hannun YA, Linardic CM. Sphingomyelin breakdown products: antiproliferative and tumor suppressor lipids. Biochim Biophys Acta 1994;1154:223–36. [DOI] [PubMed] [Google Scholar]
- [45].Wolff RA, Dobrowsky RT, Bielawski A, Obeid LM, Hannun YA. Role of ceramide-activated protein phosphatase in ceramide mediated signal transduction. J Biol Chem 1994;269:19607–9. [PubMed] [Google Scholar]
- [46].Leikin AI, Brenner RR. Fatty acid desaturase activities are modulated by phytosterol incorporation in microsomes. Biochim Biophys Acta 1989;1005:187–91. [DOI] [PubMed] [Google Scholar]
- [47].Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res 1985;26:1015–35. [PubMed] [Google Scholar]
- [48].Shinitzky M, Inbar M. Differences in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Molec Biol 1974;85:603–15. [DOI] [PubMed] [Google Scholar]
- [49].Fourcade A, Billard C, Tapiero H. Membrane dynamics of Friend Leukaemic cells I. Changes associated with cell growth. Cell Differentiation 1980;9:203–10. [DOI] [PubMed] [Google Scholar]
- [50].Tapiero H, Fourcade A, Billard C. Membrane dynamics of Friend Leukaemic cells II. Changes associated with cell differentiation. Cell Differentiation 1980;9:211–8. [DOI] [PubMed] [Google Scholar]
- [51].Shmeeda HR, Golden EB, Barenholz Y. Membrane lipids and aging. In: Shinitzky M, editor. Biomembranes: structural and functional aspects Weinhein (Germany: ): VCH; 1994. p. 1–82. [Google Scholar]