Figure 1.
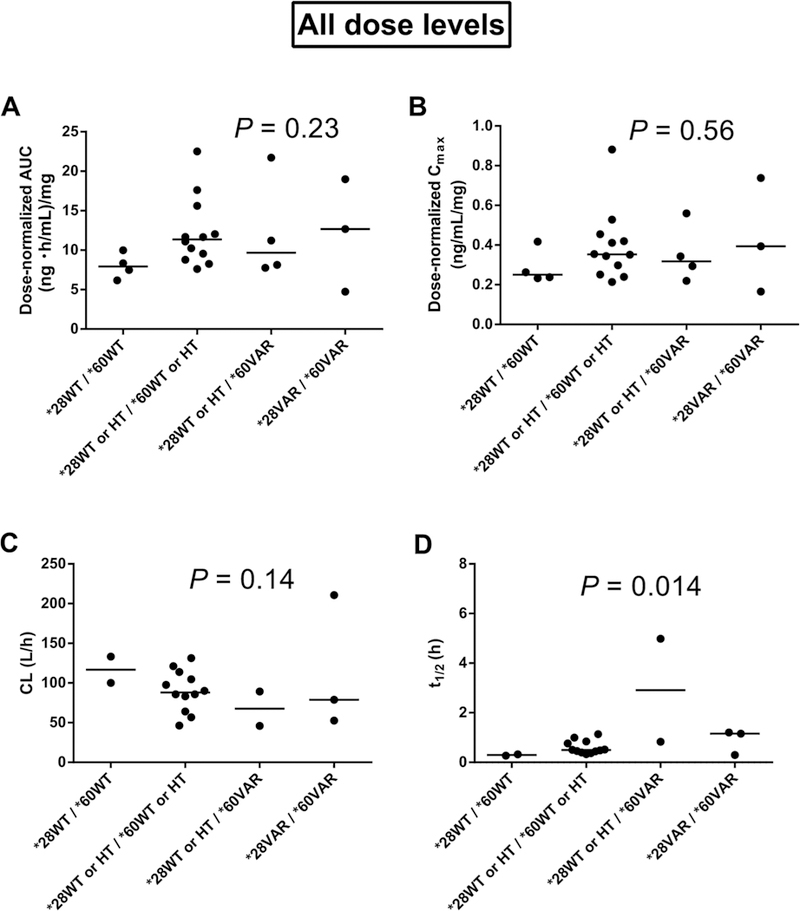
Effects of combined variants of uridine diphosphate glucuronosyltransferase I family, polypeptide A I (UGTIAI)*28 and *60 on belinostat pharmacokinetics for all dose levels in the trial of belinostat (400, 500, 600, or 800 mg/m2/24 h, 48-hour continuous infusion) in combination with cisplatin and etoposide. (A) UGT IA I vs dose-normalized area under the plasma concentration-time curve (AUC, n = 23). (B) UGTIAI vs dose normalized maximum plasma concentration (Cmax, n = 23). (C) UGTIAI versus total body clearance (CL, n = 19). (D) UGTIAI versus elimination halflife (t1/2, n = 19). P values were obtai ned using the Jonckheere-Terpstra test for trend (α =.0I required for significance). WT, wild type; HT, heterozygous; VAR, homozygous.
