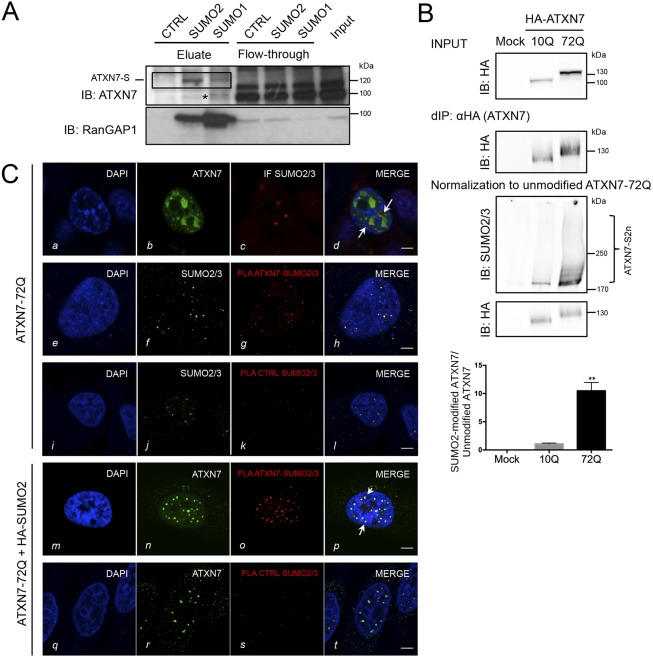
Fig. 1.
ATXN7 is modified by SUMO2 in cells. (A) MCF7 cell lysate was subjected to denaturing immunoprecipitations with beads coupled to monoclonal antibodies against SUMO1, SUMO2 or IgG (control). Top: enriched endogenous SUMO targets were eluted from beads with peptides corresponding to the epitopes of both SUMO antibodies. Shown are immunoblots against ATXN7 and against the abundant SUMO target RanGAP1 as positive control. SUMO-modified ATXN7 is boxed (ATXN7-S). The asterisk indicates non-specific band. (B) Both wild-type (10Q) and mutant (72Q) ATXN7 are SUMO2/3 modified. HEK293 cells expressing HA-ATXN7 with 10Q or 72Q were subjected to denaturing immunoprecipitation (d-IP) using anti-HA antibody-coupled beads (d-IP: HA), followed by western blotting. Input and d-IP products are revealed with anti-HA tag (top). To compare the level of SUMO2/3 modification, normalization to the unmodified protein is mandatory: d-IP products with a similar level of unmodified HA-ATXN7-10Q and 72Q were analyzed (IB: HA, bottom). Quantification of the SUMOylated species is shown (graph). Results are mean±s.d. Statistical analysis was performed using Student's t-test (**P<0.01; n=3). (C) Interaction between ATXN7-72Q and SUMO2/3 and their colocalization in HeLa cells determined by proximity ligation assay (PLA, antibodies used are written in red) and immunofluorescence. Row a-d: overexpressed ATXN7-72Q detected by immunofluorescence appears as irregular dots; some of them are colocalized or juxtaposed (arrows) with endogenous SUMO2/3. Row e-h: overexpressed ATXN7 and endogenous SUMO2/3: the red dots in the PLA assay demonstrate that mutant ATXN7 and endogenous SUMO2/3 are in close contact. By immunofluorescence (green dots), we detected ATXN7 and SUMO2/3. Row m-p: co-expression of mutant ATXN7-72Q and SUMO2 led to the complete colocalization between ATXN7 dots (green, immunofluorescence) and PLA foci (red). Arrows in p indicate examples of complete colocalization between ATXN7 immunofluorescence and the PLA signal. Mouse monoclonal anti-1C1 (ATXN7) and rabbit polyclonal anti-SUMO2/3 antibodies were used. Rows i-l and q-t: two negative controls for PLA show that no unspecific signal was detected (k, s). Representative confocal images are shown. Scale bars: 5 µm.