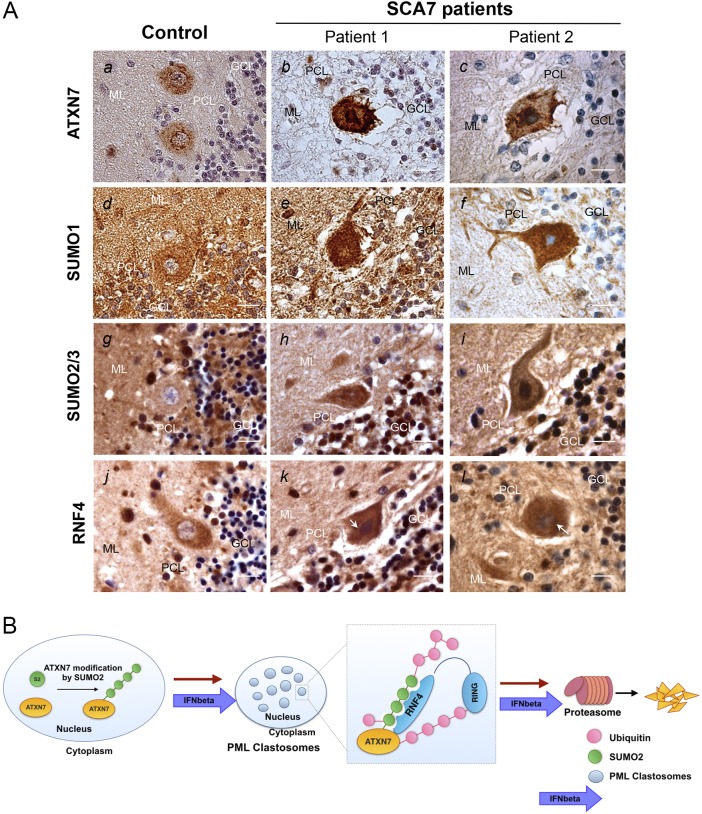
Fig. 7.
SUMO proteins accumulate abnormally in the cerebellum of two SCA7 patients. Model proposed for mutant ATXN7 degradation via the proteasome. (A) Postmortem cerebellar tissues from two SCA7 patients with morphologically and genetically confirmed SCA7 (57 and 10 years old at death; with 47 CAG repeats and 85 CAG repeats on the mutant allele in peripheral blood, respectively) and a control (52 year-old) with no neurological disease were analyzed by immunohistochemistry; representative images are shown. In the cerebellum of the SCA7 patients, ATXN7 accumulated strongly in nuclei and cytoplasm of the few surviving Purkinje cells (b, c). SUMO1 (e, f) and SUMO2/3 (h, i) immunoreactivity increased strongly in the nucleus and, to a lesser extent, in the cytoplasm of Purkinje cells in the SCA7 patients. RNF4 accumulated in the SCA7 patients, both in the cytoplasm and in the nucleus of the Purkinje cells (k, l). We analyzed n=20 Purkinje cells for each patient: cells in which nuclei were not visible were not taken into account. GCL, granular cell layer; ML, molecular layer; PCL, Purkinje cell layer. Scale bars: 20 μm. (B) Model for mutant ATXN7 degradation via the proteasome. We propose this model, which summarizes data from this study and our previously published results on polyQ-ATXN7 degradation by PML clastosomes and their stimulation by IFN-beta treatment (Janer et al., 2006; Chort et al., 2013). First, mutant ATXN7 is modified by SUMO2 by addition of poly-SUMO2/3 chains. This modification promotes the recruitment of ATXN7 to PML clastosomes, where it is ubiquitinated by the SUMO-dependent ubiquitin ligase RNF4 for its final degradation by the proteasome. Treatment with IFN-beta enhances clastosome formation and increases SUMO pathway enzymes, promoting further poly-SUMOylation by SUMO2/3 of polyQ-expanded ATXN7, which finally leads to its enhanced clearance.