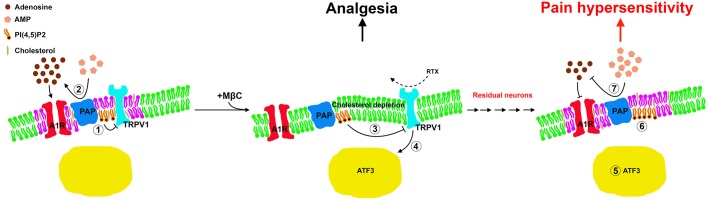
Fig. 8.
Antinociceptive dysfunction by disrupting of cholesterol-rich membrane microdomains with MβC in RTX neuropathy. Cholesterol-rich membrane microdomains act as novel units for pain modulation in RTX neuropathy through the following mechanisms. (Left panel) In a silent neuron, TRPV1 colocalizes with adenosine signaling molecules such as PAP and A1R in a specific cholesterol-rich membrane microdomains (labeled in purple; rest of the plasma membrane labeled in green), resulting in (1) hydrolyzing PI(4,5)P2 that consequently reduces TRPV1 sensitization and (2) AMP hydrolyzing to adenosine, resulting in antinociception. (Middle panel) (3) At the acute stage of RTX neuropathy, MβC disrupted the microdomain by depleting cholesterol, which preserves PAP-hydrolyzed PI(4,5)P2 and antinociception despite (4) TRPV1 sensitization and ATF3 upregulation following RTX administration. (Right panel) At the chronic stage, depletion of TRPV1(+) neurons is mildly associated with PAP(+) and A1R(+) neuron depletion; moreover, (5) the residual A1R(+) and PAP(+) neurons are associated with ATF3 upregulation, (6) suppression of PI(4,5)P2 hydrolysis, and (7) reduced availability of adenosine (Kan et al., 2018), which consequently induces pain hypersensitivity.