Abstract
Background
The oncogenic potential of special AT-rich binding protein 1 (SATB1) has been reported in various types of cancer, but its function in cervical cancer remains not fully investigated. This study aimed to investigate the effect of SATB1 mRNA expression on tumor progression and outcomes in the cervical cancer patients.
Methods
A total of 33 cervical cancer patients treated in our hospital from September 2012 to December 2015 were included. The mRNA expression level of STAB1 in cervical cancer tissue was determined by real-time PCR, and the patients were divided into dichotomous groups based on their SATB1 expression level. Clinical characteristics, recurrence, and survival outcomes were compared between groups.
Results
Compared with the SATB1-low group, the SATB1-high group had significantly advanced International Federation of Gynecology and Obstetrics (FIGO) stages (P=0.037) and histologic grade (P=0.036). Kaplan–Meier analysis showed that SATB1-high group had a worse overall survival (P=0.078, marginal significant). In the subgroup analysis of pathological types, adenocarcinomas group (n=8) had a significantly higher SATB1 expression level as compared with the squamous cell carcinomas (n=18) and adenosquamous carcinomas (n=7) groups (both P<0.05). Cervical squamous cell carcinomas patients with a high-expression SATB1 (n=8) had more advanced FIGO stages (P=0.015) and histologic grades (P=0.060, marginal significant) as well as a higher (P=0.069, marginal significant) incidence of lymphatic metastasis than those with a low expression of SATB1 (n=10).
Conclusion
These results showed that expression of SATB1 may have an effect on the disease progression and survival outcome of cervical cancer.
Keywords: cervical cancer, special AT-rich sequence-binding protein 1, SATB1, prognosis
Introduction
Cervical cancer is the third most common malignancy and the fourth leading cause of cancer death among women worldwide.1 Approximately 85% of cervical cancers and deaths occur in low- and middle-income countries.2 In China, ~88,000 newly diagnosed cases and 23,000 deaths were estimated in 2011.3 Cervical cancer is usually asymptomatic in the early stages, but may cause symptoms including abnormal vaginal bleeding, discharge, pelvic pain, and dyspareunia in the locally advanced disease.4 The 5-year survival rate is 88%–95% in the patients with International Federation of Gynecology and Obstetrics (FIGO) stages IB–IIA cervical cancer without lymph node metastasis but drops to 51%–78% in those without lymph node metastasis.5 The relapse rate of cervical cancer is around 11%–22% in the FIGO stages IB–IIA, but elevated to 28%–64% in the stages IIB–IVA.6 Once recurrence, the 5-year survival rate is considerably reduced to 22.3%.7 Thus, identification of specific tumor-related marker may improve the early diagnosis rate and the prognosis of cervical cancer.
At present, several proteins have been identified as the biomarkers for invasive cervical cancer, such as p16ink4a, p16, E-cadherin, Ki67, pRb, p53, CEA, SCC-Ag, and CD44.8 Vitale et al have demonstrated that p16NK4a expression is a marker of disease progression in the patients with a low-grade squamous intraepithelial lesion in cervical cytology.9 In addition to protein markers, miRNAs have also been shown to be differentially expressed in endometrial lesions, such as endometrial cancer, endometriosis, and chronic endometritis.10,11 For example, Di Pietro et al have reported that miR-27a-3p and miR-124-3 p are upregulated in the endometrium and serum of patients with chronic endometritis, which could be used as potential molecular markers of endometritis.12 It has been shown that miR-424-5 p, miR-9-5 p, miR-133b, and miR-200a-5p are involved in the pathogenesis of cervical cancer by targeting the key proteins in the cell cycle (such as CHEK1 and CDKN2A) and cell transformation (such as SOX17).13
Special AT-rich binding protein 1 (SATB1) is a global chromatin remodeling protein which recruits chromatin remodeling proteins to epigenetically regulate the expression of a large number of genes.14,15 It is known that SATB1 plays a role in a variety of important biological processes, such as postnatal cerebral cortical development,16 T-cell development,17 lymphocyte differentiation,18 and X-chromosome inactivation.19 In addition to these physiological functions, SATB1 has been shown to be associated with various types of cancers, including esophageal squamous cell carcinoma,20 colorectal,21 pancreatic,22 lung,23 liver,24 kidney,25 and ovarian26 cancers. Altered expression of SATB1 has also been shown to be associated with the development of gynecological cancers. Zhang et al have shown that SATB1 is expressed in 45.3% of endometrial cancer tissues, but not in the normal endometrial tissues.27 SATB1 is an independent factor associated with overall survival (HR =2.928) and disease-free survival (HR =2.825).27 On the other hand, high SATB1 expression provides benefit for estrogen receptor-positive breast cancers.28
Currently, the study on the prognostic value of SATB1 expression in cervical cancer is still extremely rare. Wang et al have recently investigated the association between the SATB1 expression (by immunohistochemistry) and outcomes in cervical squamous cell carcinoma (CSCC). Their results showed that SATB1 expression was associated with tumor progression, metastasis, and poor prognosis in CSCCs. However, their study focused only on CSCCs, so that the effect of SATB1 expression on the other pathological types of cervical cancers, such as cervical adenocarcinomas, remains unknown. Therefore, the purpose of this study was to investigate the effect of SATB1 mRNA expression on the tumor progression and outcomes of cervical cancer patients.
Methods
Patients
A total of 33 patients pathologically diagnosed with cervical cancer in the Department of Gynecology, the Maternal and Child Health Hospital of Foshan from September 2012 to December 2015 were included. The cervical cancer patients who received any preoperative hormone therapy, chemotherapy, or radiotherapy were excluded. All the selected subjects had no other tumors. The stage of the cervical cancer was determined according to the FIGO staging system,29 and the histologic grades of cancers were assessed based on the pathological examination of paraffin-embedded sections. This study was approved by the institutional review board of Maternal and Child Health Hospital of Foshan (No FSFY-MEC-2015-001) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient.
Sample preparation and RNA extraction
Cervical tissue samples were obtained from all the cases by surgical resection and were immediately stored in liquid nitrogen until RNA extraction. Total tissue RNA was extracted using TRIZOL reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. RNA samples were dissolved in diethylpyrocarbonate (DEPC)-treated water and stored at -80°C until further use. The quality of total RNA extraction was validated with an OD260/OD280 ratio of 1.8:2.0 and the presence of intact bands of 18S and 28S ribosomal RNAs in agarose gel (1%) electrophoresis.
Real-time quantitative RT-PCR
All primers were designed using the Primer Express 2.0 software (ABI Prism; PE Biosystems, Foster City, CA, USA). During designing primers, the factors affecting PCR efficiency, such as the annealing temperature and the length of the amplified fragment, should be kept consistent to guarantee the PCR efficiency. GAPDH is one of the commonly used reference gene for PCR, and its expression has not been reported to interfere with the target gene(s). Therefore, GAPDH was chosen as a reference gene. For quantitative real-time RT-PCR, single-stranded cDNAs were generated using Prime Script™ RT reagent Kit (cat no RR047A; Takara, Kusatsu, Japan) according to the manufacturer’s instruction. PCR was performed using the reaction mixture (20 µL) as follows: 2.0 µL cDNA, 6 µM of each primer, 10 µL (2×) SYBR™ Premix Dimer Eraser (cat no RR820A; Takara), and 8.5 µL deionized water. The thermal cycling conditions were as follows: 94°C for 3 minutes (one cycle), 94°C for 5 seconds, and 60°C for 30 seconds (40 cycles), followed by 95°C for 15 seconds, 60°C for 30 seconds, 94°C for 15 seconds (one cycle). Duplicate PCR amplifications were performed for each sample. The amount of target gene expression was extrapolated from the standard curve and normalized to GAPDH (internal control). The relative expression of SATB1 was determined by the 2−ΔΔCt method. Among the 33 patients, the case with the minimal SATB1 expression level (the maximum Ct value) was used as a control (with an expression amount of 1). Relative expression levels were then calculated from Ct values using the formula: 2−ΔΔCt, where −ΔΔCt = each corresponding Ct value - the maximum Ct value. Primer sequences for quantitative RT-PCR were as follows: human-SATB1 (amplified fragment length =121 bp): forward-5′-ACCCTGGGCTCGTATCAACA-3′, reverse-5′-TGGTTTAAGGACTGCTGGGC-3′; human-GAPDH (internal control, amplified fragment length =95 bp): forward-5′-GATTCCACCCATGGCAAATT-3′, reverse-5′-TCTCGCTCCTGGAAGATGGT-3′.
Statistical analysis
Continuous data were presented as mean ± SD. Mean were compared by Student’s independent t-test or one-way ANOVA and Fisher’s least significant difference (LSD) post hoc tests. If normality of continuous data was not assumed, Mann–Whitney U test or Kruskal–Wallis test would be used instead. Categorical data were indicated with number and percentage and were compared by chi-squared test or Fisher’s exact test if the expected value of <5 was found. Kaplan–Meier survival analysis was used to investigate the association between the independent variables and the overall survival/progression-free survival. All statistical analyses were performed using IBM SPSS Version 20 (IBM Corporation, Armonk, NY, USA). Significance level was set at P<0.05, two-tailed.
Results
Patient’s clinical characteristics and outcomes
A total of 33 cervical cancer patients (mean age =48.27± 9.30 years) were included. The demographic and clinical characteristics, as well as the outcomes of the patients, were summarized in Table 1. Three pathological types were found in the cervical cancer group, including squamous cell carcinomas (54.5%), adenocarcinomas (24.2%), and adenosquamous carcinomas (21.2%). As for the FIGO stages, 18 (54.5%) and 15 (45.5%) patients were at stage I and stage II, respectively. Regarding histologic grades, two (6.1%), 25 (75.8%), and six (18.2%) patients were at G1, G2, and G3, respectively. Ten (30.3%) cervical cancer patients had lymphatic metastasis. The mean followed-up time for the cervical cancer group was 21.67±8.25 months (median =22, range: 15–28 months). Three (9.1%) cases died during the follow-up and six (18.2%) patients suffered from a recurrence, including three local recurrences, two distant metastases, and one local recurrence + distant metastasis.
Table 1.
Patient’s clinical characteristics and outcomes
| Parameters | Cervical cancer (n=33) |
|---|---|
|
| |
| Clinical characteristics | |
| Age, year, mean±SD | 48.27±9.30 |
| Pathological type, n (%) | |
| Squamous cell carcinoma | 18 (54.5) |
| Adenocarcinoma | 8 (24.2) |
| Adenosquamous carcinoma | 7 (21.2) |
| FIGO stage, n (%) | |
| Stage I | 18 (54.5) |
| Stage II | 15 (45.5) |
| Stage III | 0 |
| Histologic grade, n (%) | |
| G1 | 2 (6.1) |
| G2 | 25 (75.8) |
| G3 | 6 (18.2) |
| Lymphatic metastasis | 10 (30.3) |
| SATB1 expression levels | 4.91±1.85 |
| Clinical outcomes, n (%) | |
| Death | 3 (9.1) |
| Progression | 6 (18.2) |
| Recurrence, n (%) | |
| No | 27 (81.8) |
| Local recurrence | 3 (9.1) |
| Distant metastasis | 2 (6.1) |
| Local recurrence and distant metastasis | 1 (3.0) |
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
High SATB1 expression was associated with advanced stages of cancers
The mRNA expression level of STAB1 was determined by real-time PCR and presented as a relative value using the case with the minimal expression level as a control (with an expression amount of 1). To address if STAB1 gene plays a role in the pathogenesis of cervical cancer, the 33 patients were dichotomously divided into the SATB1-high group (n=17) and the SATB1-low group (n=16) using the median of relative expression of SATB1 gene (median: 4.77) of the 33 patients as the cutoff. As shown in Table 2, the SATB1-high group had significantly more advanced FIGO stage (P=0.037) and histologic grade (P=0.036) as compared with the SATB1-low group.
Table 2.
Comparison of characteristics and outcomes in the groups stratified by the SATB1 expression level
| Parameters | SATB1-low (n=16) | SATB1-high (n=17) | P-value |
|---|---|---|---|
|
| |||
| Clinical characteristics | |||
| Age, year, mean±SD | 48.75±10.69 | 47.82±8.09 | 0.928 |
| Pathological type, n (%) | 0.297 | ||
| Squamous cell carcinoma | 10 (62.5) | 8 (47.1) | |
| Adenocarcinoma | 2 (12.5) | 6 (35.3) | |
| Adenosquamous carcinoma | 4 (25.0) | 3 (17.6) | |
| FIGO stage, n (%) | 0.037 | ||
| Stage I | 12 (75.0) | 6 (35.3) | |
| Stage II | 4 (25.0) | 11 (64.7) | |
| Stage III | 0 | 0 | |
| Histologic grade, n (%) | 0.036 | ||
| G1 | 0 | 2 (11.8) | |
| G2 | 15 (93.8) | 10 (58.8) | |
| G3 | 1 (6.3) | 5 (29.4) | |
| Lymphatic metastasis | 3 (18.8) | 7 (41.2) | 0.161 |
| SATB1 expression levels | 3.49±0.78 | 6.24±1.54 | <0.001 |
| Clinical outcomes, n (%) | |||
| Death | 0 | 3 (17.6) | 0.227 |
| Progression | 1 (6.3) | 5 (29.4) | 0.175 |
| Recurrence, n (%) | 0.187 | ||
| No | 15 (93.8) | 12 (70.6) | |
| Local recurrence | 1 (6.3) | 2 (11.8) | |
| Distant metastasis | 0 | 2 (11.8) | |
| Local recurrence and distant metastasis | 0 | 1 (5.9) | |
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
High SATB1 expression was associated with worse survival outcomes
The survival outcomes were also compared between groups. As shown in Table 2, the SATB1-high group had a relatively higher death rate, progression rate, and recurrence rate than the SATB1-low group, even though the difference did not reach significance (all P>0.05). Kaplan–Meier survival curve analysis showed that SATB1-high group had a worse overall survival (log-rank test, P=0.078, Figure 1) and a worse progression-free survival as compared with the SATB1-low group (log-rank test, P=0.106, Figure 2). Although no significance was found, the difference in overall survival reached marginal significance (P=0.078).
Figure 1.
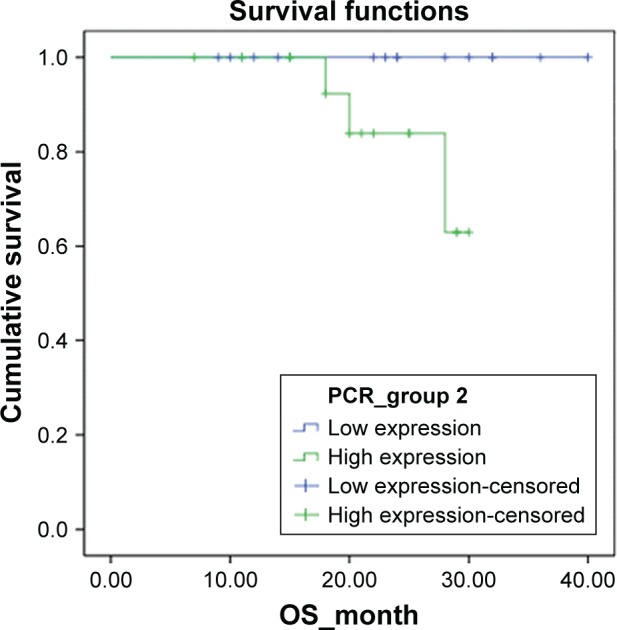
Kaplan–Meier survival function of overall survival (OS) for the dichotomous groups stratified by the mRNA level of SATB1 gene.
Notes: SATB1-high group vs SATB1-low group, log-rank-test, P=0.078.
Figure 2.
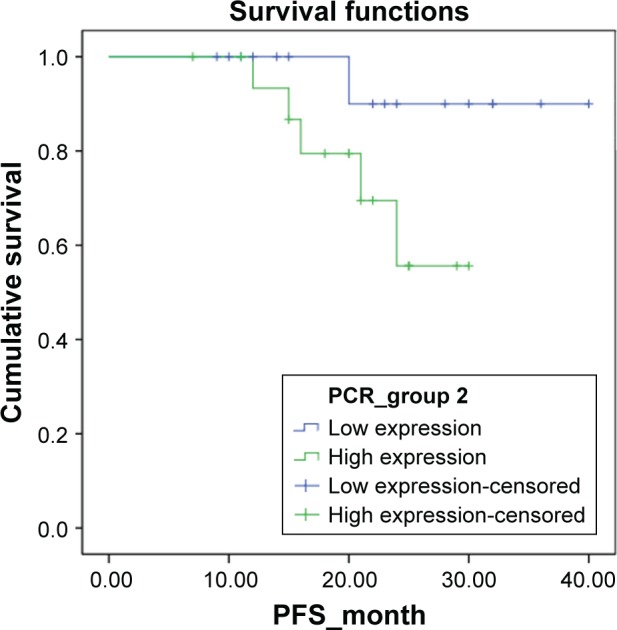
Kaplan–Meier survival function of progression-free survival (PFS) for the dichotomous groups stratified by the mRNA level of SATB1 gene.
Notes: SATB1-high group vs SATB1-low group, log-rank-test, P=0.106.
Analysis stratified by the pathological types of cervical cancer
Next, we attempted to address if the expression level of SATB1 is associated with the pathological types of cervical cancer. The patients were divided into three groups according to their pathological types of cancer. As shown in Table 3, adenocarcinomas group (n=8) had a significantly higher SATB1 expression level as compared with the squamous cell carcinomas (n=18) and adenosquamous carcinomas (n=7) groups in LSD post hoc comparisons (both P<0.05).
Table 3.
Comparison of characteristics and outcomes in the three groups stratified by the pathological types of cervical cancer
| Parameters | Squamous cell carcinoma (n=18) | Adenocarcinoma (n=8) | Adenosquamous carcinoma (n=7) | P-value |
|---|---|---|---|---|
|
| ||||
| Clinical characteristics | ||||
| Age, year, mean±SD | 48.83±9.19 | 42.13±6.88 | 53.86±8.90 | 0.082 |
| FIGO stage, n (%) | 0.375 | |||
| Stage I | 9 (50.0) | 6 (75.0) | 3 (42.9) | |
| Stage II | 9 (50.0) | 2 (25.0) | 4 (57.1) | |
| Stage III | 0 | 0 | 0 | |
| Histologic grade, n (%) | 0.603 | |||
| G1 | 1 (5.6) | 1 (12.5) | 0 | |
| G2 | 15 (83.3) | 5 (62.5) | 5 (71.4) | |
| G3 | 2 (11.1) | 2 (25.0) | 2 (28.6) | |
| Lymphatic metastasis | 3 (16.7) | 3 (37.5) | 4 (57.1) | 0.128 |
| SATB1 expression levels | 4.42±1.56 | 6.13±2.44* | 4.76±1.32 | 0.089 |
| Clinical outcomes, n (%) | ||||
| Death | 0 | 1 (12.5) | 2 (28.6) | 0.058 |
| Progression | 1 (5.6) | 2 (25.0) | 3 (42.9) | 0.082 |
| Recurrence, n (%) | 0.103 | |||
| No | 17 (94.4) | 6 (75.0) | 4 (57.1) | |
| Local recurrence | 1 (5.6) | 1 (12.5) | 1 (14.3) | |
| Distant metastasis | 0 | 0 | 2 (28.6) | |
| Local recurrence and distant metastasis | 0 | 1 (12.5) | 0 | |
Notes:
The SATB1 level of adenocarcinoma patients was significantly higher than the other two groups (P<0.05) in LSD post hoc comparisons.
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Subgroup analysis in the CSCCs
To further investigate the effect of SATB1 expression on the CSCCs, the 18 CSCC patients were subdivided into dichotomous groups based on their expression level of SATB1. The results showed that CSCC patients with a high-expression SATB1 (n=8) had a significantly advanced FIGO stage of disease as compared with those with a low expression of SATB1 (n=10, P=0.015). Meanwhile, CSCC patients with a high expression of SATB1 also had with a more advanced histologic grade (P=0.060) and a higher (P=0.069) incidence of lymphatic metastasis. Both the differences reached marginal significance.
Discussion
In this study, we investigated the effect of SATB1 expression on the tumor stages and outcomes of cervical cancer patients. The result showed that compared with the patients with low SATB1 expression, those with high SATB1 expression had significantly more advanced FIGO stages (P=0.037) and histologic grade (P=0.036) of cancers. Kaplan–Meier analysis showed that SATB1-high group had a worse overall survival (P=0.078, marginally significant). In the subgroup analysis of pathological types, adenocarcinomas group (n=8) had a significantly higher SATB1 expression level as compared with the squamous cell carcinomas (n=18) and adenosquamous carcinomas (n=7) groups (both P<0.05). Subgroup analysis in CSCC showed that patients with high SATB1 expression (n=8) had significantly more advanced FIGO stages (P=0.015) and histologic grades (P=0.060, marginally significant) as well as a higher incidence of lymphatic metastasis (P=0.069, marginally significant) than those with low SATB1 expression (n=10). Taken together, our results suggested that expression of SATB1 may have an effect on the disease progression and survival outcomes of cervical cancer.
The oncogenic potential of SATB1 has been observed in various types of malignant tumors. However, the effect of SATB1 expression in cervical cancer is rarely reported. Our study showed that high expression of SATB1 was associated with more advanced stages of cancers and a worse overall survival outcome in cervical cancer, suggesting an oncogenic potential of SATB1 gene in cervical cancer. Although the differences in lymphatic metastasis, death rate, cancer progression, and recurrence between the STAB1-high group and the STAB1-low group did not reach significance, it should be attributed to the small sample size of our study. Therefore, in the future, a large sample size study should be conducted to validate our findings. Since CSCCs is the most frequent type of cervical cancer (accounting for 70%–80% of all cervical cancers),4 our observations in different pathological types of cervical cancers are consistent with Wang et al’s study on CSCC.30 Their results showed that CSCC patients with positive SATB1 expression have advanced FIGO stage, histologic grade, and lymph node metastasis, as well as a worse overall survival and disease-free survival.30 Moreover, our subgroup analysis in CSCCs also confirmed that high SATB1 expression was associated with stages and lymphatic metastasis, which is also in line with Wang et al’s study.
In addition to these consistent findings, our study further found that high expression of SATB1 was more frequently observed in cervical adenocarcinomas than CSCCs and cervical adenosquamous carcinomas. Cervical adenocarcinomas are the second most frequent type of cervical cancers, accounting for 10%–15% of all cases.4 However, due to the small sample size of the cervical adenocarcinomas (n=7) in the current study, we cannot further conduct stratified analysis based on the expression levels of SATB1 within the cervical adenocarcinomas patients. Since differential expression levels of SATB1 were observed among different pathological types of cervical cancers, further investigation is necessary to investigate if SATB1 exhibits different oncogenic potential among the different pathological types.
Some studies have reported the molecular mechanism for the oncogenic potential of STAB1 in other cancers. For instance, in the microarray analysis, Han et al have demonstrated that SATB1 knockdown in MDA-MB-231 cells (a highly aggressive breast cancer cell line) changes the expression of >1,000 genes and reverses the tumorigenicity in vivo.31 Their study further showed that SATB1 directly upregulates the genes associated with metastasis, angiogenesis, and breast cancer progression, such as metastasin, matrix metalloproteases 2, 3, and 9, transforming growth factor-b1 (TGFB1), epidermal growth factor receptor (EGFR) family, and downregulates the metastasis suppressor genes (such as BRMS1 and KAI1) and epithelial to mesenchymal transition-associated gene (E-cadherin).31 Sun et al have reported that overexpression of SATB1 in Michigan Cancer Foundation-7 (MCF-7) breast cancer cells helps to maintain the characteristics of breast cancer stem cells by activating the Notch signaling.32 Recently, a whole genome transcriptome analysis by Song et al have revealed that fibronectin 1 and platelet-derived growth factor receptor, beta polypeptide are the key downstream genes regulated by SATB1 in esophageal cancer cells, and SATB1 exerts an oncogenic effect in esophageal cancer by upregulation of these two genes.33 It is worth to further investigate if the oncogenic function of STAB1 in cervical cancer is involved in these molecular mechanisms.
Several limitations of this study should be pointed out. First, this study was limited by the small sample size and its retrospective nature. In the future, a well-designed prospective study with a large sample size should be conducted to validate the findings of the current study. In addition, although we assessed the mRNA expression level of STAB1, we did not further determine the STAB1 protein expression level in these specimens. Moreover, we did not simultaneously analyze the surrounding normal tissues of the cervical cancer specimens to address if there was differential expression of SATB1 between normal and cancer tissues. All these limitations should be addressed in future studies.
Conclusion
Our results showed that high expression of SATB1 was associated with more advanced stages of cervical cancers and worse overall survival in cervical cancer patients. Cervical adenocarcinomas were associated with a high expression of SATB1. Our findings should aid the understanding of the prognostic value of SATB1 in cervical cancer, but further study should be conducted to validate these findings.
Acknowledgments
This study was supported by the Science and Technology Commission of Guangdong Province, China (2015A020210002).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Campos NG, Sharma M, Clark A, Kim JJ, Resch SC. Resources required for cervical cancer prevention in low- and middle-income countries. PLoS One. 2016;11(10):e0164000. doi: 10.1371/journal.pone.0164000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34(11):502–507. doi: 10.1186/s40880-015-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo N, Carinelli S, Colombo A, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii27–vii32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 5.Kim SM, Choi HS, Byun JS. Overall 5-year survival rate and prognostic factors in patients with stage IB and IIA cervical cancer treated by radical hysterectomy and pelvic lymph node dissection. Int J Gynecol Cancer. 2000;10(4):305–312. doi: 10.1046/j.1525-1438.2000.010004305.x. [DOI] [PubMed] [Google Scholar]
- 6.Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. Int J Gynecol Obstet. 2006;95:S43–S103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 7.Qiu JT, Abdullah NA, Chou HH, et al. Outcomes and prognosis of patients with recurrent cervical cancer after radical hysterectomy. Gynecol Oncol. 2012;127(3):472–477. doi: 10.1016/j.ygyno.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Valenti G, Vitale SG, Tropea A, Biondi A, Laganà AS. Tumor markers of uterine cervical cancer: a new scenario to guide surgical practice? Updates Surg. 2017;69(4):441–449. doi: 10.1007/s13304-017-0491-3. [DOI] [PubMed] [Google Scholar]
- 9.Vitale SG, Valenti G, Rapisarda AM, et al. P16INK4a as a progression/regression tumour marker in LSIL cervix lesions: our clinical experience. Eur J Gynaecol Oncol. 2016;37(5):685–688. [PubMed] [Google Scholar]
- 10.Ferlita A, Battaglia R, Andronico F, et al. Non-coding RNAs in endometrial physiopathology. Int J Mol Sci. 2018;19(7):2120. doi: 10.3390/ijms19072120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He Y, Lin J, Ding Y, et al. A systematic study on dysregulated microRNAs in cervical cancer development. Int J Cancer. 2016;138(6):1312–1327. doi: 10.1002/ijc.29618. [DOI] [PubMed] [Google Scholar]
- 12.Di Pietro C, Caruso S, Battaglia R, et al. MiR-27a-3p and miR-124-3p, upregulated in endometrium and serum from women affected by chronic endometritis, are new potential molecular markers of endometrial receptivity. Am J Reprod Immunol. 2018;80(3):e12858. doi: 10.1111/aji.12858. [DOI] [PubMed] [Google Scholar]
- 13.Zhao L, Zhang Z, Lou H, et al. Exploration of the molecular mechanisms of cervical cancer based on mRNA expression profiles and predicted microRNA interactions. Oncol Lett. 2018;15(6):8965–8972. doi: 10.3892/ol.2018.8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brocato J, Costa M. SATB1 and 2 in colorectal cancer. Carcinogenesis. 2015;36(2):186–191. doi: 10.1093/carcin/bgu322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohwi-Shigematsu T, Poterlowicz K, Ordinario E, Han HJ, Botchkarev VA, Kohwi Y. Genome organizing function of SATB1 in tumor progression. Semin Cancer Biol. 2013;23(2):72–79. doi: 10.1016/j.semcancer.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balamotis MA, Tamberg N, Woo YJ, et al. SATB1 ablation alters temporal expression of immediate early genes and reduces dendritic spine density during postnatal brain development. Mol Cell Biol. 2012;32(2):333–347. doi: 10.1128/MCB.05917-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez JD, Yasui DH, Niida H, Joh T, Loh DY, Kohwi-Shigematsu T. The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev. 2000;14(5):521–535. [PMC free article] [PubMed] [Google Scholar]
- 18.Yokota T, Kanakura Y. Role of tissue-specific AT-rich DNA sequence-binding proteins in lymphocyte differentiation. Int J Hematol. 2014;100(3):238–245. doi: 10.1007/s12185-014-1602-2. [DOI] [PubMed] [Google Scholar]
- 19.Agrelo R, Souabni A, Novatchkova M, et al. SATB1 defines the developmental context for gene silencing by Xist in lymphoma and embryonic cells. Dev Cell. 2009;16(4):507–516. doi: 10.1016/j.devcel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Wu K, Zhao Z, Miao R, Xu Z. Special AT-rich sequence binding protein 1 promotes tumor growth and metastasis of esophageal squamous cell carcinoma. Tumour Biol. 2017;39(3):101042831769453. doi: 10.1177/1010428317694537. [DOI] [PubMed] [Google Scholar]
- 21.Mansour MA, Hyodo T, Akter KA, et al. SATB1 and SATB2 play opposing roles in c-myc expression and progression of colorectal cancer. Oncotarget. 2016;7(4):4993–5006. doi: 10.18632/oncotarget.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Li Z, Li W, et al. SATB1 promotes pancreatic cancer growth and invasion depending on Myc activation. Dig Dis Sci. 2015;60(11):3304–3317. doi: 10.1007/s10620-015-3759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selinger CI, Cooper WA, Al-Sohaily S, et al. Loss of special AT-rich binding protein 1 expression is a marker of poor survival in lung cancer. J Thorac Oncol. 2011;6(7):1179–1189. doi: 10.1097/JTO.0b013e31821b4ce0. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Zeng L, Liu F, et al. Special AT-rich DNA-binding protein-1 expression is associated with liver cancer metastasis. Oncol Lett. 2016;12(6):4377–4384. doi: 10.3892/ol.2016.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalczyk AE, Krazinski BE, Godlewski J, et al. SATB1 is down-regulated in clear cell renal cell carcinoma and correlates with miR-21-5p overexpression and poor prognosis. Cancer Genomics Proteomics. 2016;13(3):209–217. [PubMed] [Google Scholar]
- 26.Nodin B, Hedner C, Uhlén M, Jirström K. Expression of the global regulator SATB1 is an independent factor of poor prognosis in high grade epithelial ovarian cancer. J Ovarian Res. 2012;5(1):24. doi: 10.1186/1757-2215-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Wang L, Liu Y, et al. Overexpression of special AT-rich sequence-binding protein 1 in endometrial cancer: a clinicopathologic study. Int J Gynecol Cancer. 2015;25(1):4–11. doi: 10.1097/IGC.0000000000000314. [DOI] [PubMed] [Google Scholar]
- 28.Hanker LC, Karn T, Mavrova-Risteska L, et al. SATB1 gene expression and breast cancer prognosis. The Breast. 2011;20(4):309–313. doi: 10.1016/j.breast.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Wang L, Zhang Y, et al. Special AT-rich sequence-binding protein 1: a novel biomarker predicting cervical squamous cell carcinoma prognosis and lymph node metastasis. Jpn J Clin Oncol. 2015;45(9):812–818. doi: 10.1093/jjco/hyv093. [DOI] [PubMed] [Google Scholar]
- 31.Han H-J, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452(7184):187–193. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z, Zhang C, Zou X, et al. Special AT-rich sequence-binding protein-1 participates in the maintenance of breast cancer stem cells through regulation of the Notch signaling pathway and expression of Snail1 and Twist1. Mol Med Rep. 2015;11(5):3235–3242. doi: 10.3892/mmr.2015.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song G, Liu K, Yang X, et al. SATB1 plays an oncogenic role in esophageal cancer by up-regulation of FN1 and PDGFRB. Oncotarget. 2017;8(11):17771–17784. doi: 10.18632/oncotarget.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]


