Abstract
Alpha-1 antitrypsin deficiency (AATD) manifests primarily as early-onset emphysema caused by the destruction of the lung by neutrophil elastase due to low amounts of the serine protease inhibitor alpha-1 antitrypsin (AAT). The current therapy involves weekly intravenous infusions of AAT-derived from pooled human plasma that is efficacious, yet costly. Gene therapy applications designed to provide constant levels of the AAT protein are currently under development. The challenge is for gene therapy to provide sufficient amounts of AAT to normalize the inhibitor level and anti-neutrophil elastase capacity in the lung. One strategy involves administration of an adeno-associated virus (AAV) gene therapy vector to the pleural space providing both local and systemic production of AAT to reach consistent therapeutic levels. This review focuses on the strategy, advantages, challenges, and updates for intrapleural administration of gene therapy vectors for the treatment of AATD.
Keywords: alpha-1 antitrypsin deficiency, gene therapy
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an autosomal recessive disorder that affects 1/1500 to 1/5000 people of European ancestry.1,2 In the United States, approximately 90,000 individuals are affected, with an additional 175,000 worldwide.1,3,4 The disease manifests primarily in the lung, presenting as early-onset emphysema and a reduced lifespan.5-11 Smoking accelerates the lung destruction process.12-14 AATD can also present as other lung diseases including bronchiectasis and asthma, and a subset of individuals develop liver cirrhosis or rarely, hepatocellular carcinoma, panniculitis and vasculitic or autoimmune disorders.4,15-23 The emphysema associated with AATD is caused by the slow destruction of the lung parenchyma by unregulated neutrophil elastase, which is released by dying or activated neutrophils.5-7,24 AAT regulates neutrophil elastase and other proteases, including proteinase 3, α-defensins, and cathepsin G, and has been shown to have anti-inflammatory properties and the ability to modulate immune responses.25-34 AAT, a serine protease inhibitor (SERPIN), mainly acts in the lower respiratory tract to inhibit the action of these proteases. AAT deficiency results in an imbalance between the proteases and AAT in the lung leading to destruction of the lung matrix and damage to the alveolar structures.5-7,24,35
AAT is a 52 kDa protein that is produced and secreted mainly from the liver into the plasma. AAT reaches the lung primarily by diffusion from the circulation,5-8,10,13,36-38 but a small amount is also produced locally by bronchial epithelial cells, mononuclear phagocytes, and neutrophils.39-43 The normal range of AAT in the serum is 20 to 53 μM; levels >11 μM are required to protect the lung from destruction.4,7,13,44,45 The low levels of AAT in AATD are caused by mutations in the SERPINA1 gene that has over 120 naturally occurring allelic variants.2,4,46-49 The normal M alleles, consisting of M1(Ala213), M1(Val213), M2, M3, and M4, are present in more than 98% of the population.50 The most prevalent deficient allele is the Z variant, which has a single amino acid substitution of lysine for glutamic acid at position 342 (E342K) that causes the polymerization of the AAT protein during post-translational processing preventing its secretion from hepatocytes.1,2,8,51-54 Homozygous Z individuals have 10%-15% of the serum AAT levels of individuals with the normal M allele and account for >95% of cases of clinically diagnosed AATD.2,5-7,13,16,38,55 The S allele, which has an amino acid substitution of valine for glutamic acid at position 264 (E264V), results in an AAT with reduced serum half-life due to instability of the protein.56-59 Homozygous S individuals have ~50% of normal AAT serum levels and are not at risk, but 15% to 20% of SZ heterozygotes have serum levels <11 μM and are at risk for disease development.60
The current therapeutic strategy to protect individuals with AATD from the development and progression of emphysema is to supplement the levels of plasma AAT above the level needed to prevent destruction by proteases. The susceptibility of the lung to destruction in the absence of AAT results from the significant portion of neutrophils that reside in the pulmonary capillaries leaving the lung at high risk from unchecked neutrophil elastase destruction.61 AAT is produced mainly in liver hepatocytes and circulates systemically, but as the predominant serine protease inhibitor, its main function is to protect the fragile alveolar structures of the lung from neutrophil elastase. The level of AAT required for protection was determined to be 11 μM in the serum based on clinical observation of the development of emphysema in AATD patients.62 AAT reaches the lung by diffusion from the circulation, and the level in the lung interstitium is ~50% of plasma levels. Further diffusion into the epithelial lining fluid (ELF) is limited by the tight junctions formed by the cells of the alveolar epithelium. The level of AAT in ELF is ~5% to 10% of plasma levels, and the level required for protection is 1.2 μM.36,62 Thus, the rationale behind the current treatment strategy is to normalize levels of AAT in the ELF and interstitium by infusing AAT into the circulation and allowing it to diffuse to the other compartments, thereby normalizing the AAT levels. As long as the AAT in the plasma is above the protective threshold of 11 μM, the alveolar compartments should receive sufficient AAT to be protected. Based on this hypothesis, the current therapy consists of weekly intravenous infusions of 60 mg/kg of AAT purified from pooled human plasma to boost the level of circulating AAT. With this protein amount, AAT levels in serum are highly increased directly after the infusion but fall to near the protective threshold after one week due to the 4.5 day half-life of AAT.62 This therapeutic strategy was approved by the Food and Drug Administration (FDA) on the basis of its biochemical efficacy of maintaining the protective level of AAT in the serum and the corresponding normalization of the AAT level and anti-neutrophil elastase capacity in the lungs.63 Clinical efficacy of this therapeutic strategy was demonstrated recently using computer tomography lung density scans at total lung capacity to validate the reduced rate of progression of lung destruction.64,65
Gene Therapy for Alpha-1 Antitrypsin Deficiency
Although AAT protein augmentation is effective at reestablishing AAT levels in plasma and lung ELF and in slowing the progression of the destructive lung disease caused by AATD,64,65 it is costly and requires weekly intravenous infusions of purified AAT from pooled human plasma. The necessary repetitive intravenous therapy derived from a human product presents problems of patient compliance and risks of allergic reactions, viral contamination, or limitations in available supply.66-68 Gene therapy to treat AATD has the possibility to alleviate all of these issues. The potential for a one-time administration reduces the burden on the patient of weekly time-consuming and invasive procedures and the associated issue of patient compliance. If effective, once gene therapy has been delivered, constant levels of AAT protein would be generated and released into the circulation, eliminating the current pattern of AAT peaks directly after infusion and AAT troughs by the end of the cycle. Thus, gene therapy offers a strategy that will persistently provide protective levels of AAT to the lung and has lower risk with fewer issues for patients and supply.
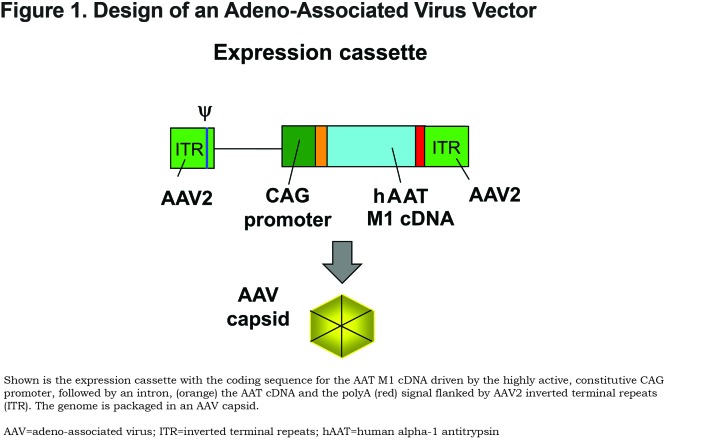
The current gene therapy strategies for AAT therapy involve delivering the normal human M allele coding sequence under the control of a highly active constitutive promoter using a gene transfer vector ( Figure 1). The goal is for transduced cells to secrete sufficient amounts of AAT into the circulation to normalize the levels of protective AAT in the lungs by diffusion after a single administration. Although a number of gene transfer vectors have been tested for delivery of the normal M-type AAT coding sequence in the past 25 years (reviewed in Chiuchiolo and Crystal61 and Sondhi et al69), the adeno-associated virus (AAV) vectors are the delivery vehicles of choice. AAV is a small parvovirus that does not cause disease in humans and causes little toxicity upon administration at doses <1015 genome copies.70,71 AAV vectors are highly effective at transducing a broad range of organs in vivo and provide persistent expression of the protein when delivered to non-proliferating cells.70 There are 6 classically described human serotypes of AAV and greater than 50 recently identified serotypes from humans and nonhuman primates (NHPs).72,73 Many AAV serotypes are available for use that have low prevalence in the general population and low or absent levels of pre-existing anti-vector immunity.72,73 Serotypes AAV1, AAV2, AAV5, AAV6, AAV8, AAV9, and AAVrh.10 have all been used in preclinical studies of AAT therapy,74-88 and clinical studies have been carried out using AAV1 and AAV2 vectors.89-91
The Case for Intrapleural Administration for Alpha-1 Antitrypsin Gene Therapy
Depending on the route of administration, the delivery of the AAT gene could allow AAT to be produced by many different cells and organs in addition to hepatocytes where the bulk of natural AAT is produced. This opens an array of possible routes of administration for a gene therapy vector, with the primary goal being to achieve the threshold levels of AAT protein of 11 μM in the serum and 1.2 μM in the alveolar ELF necessary to protect from neutrophil elastase proteolytic activity.7,13,36,62 Several different routes of administration have been attempted for AAT gene therapy. The earliest attempts targeted the respiratory tract epithelium directly, but the delivered vectors were unable to generate therapeutic levels of AAT.80,81,92,93 This failure is likely due to the natural defenses of the lung against pathogens and foreign substances, as well as the lack of viral receptors on the apical surface of respiratory epithelial cells.92-96 Routes of delivery that target the liver directly, including intravenous and intraportal administration, have been assessed in preclinical studies but have not made the transition into humans.76,85,87 Several studies have evaluated AAV vectors targeted to skeletal muscle in both preclinical animal studies and human clinical trials.75,79,84,97 A clinical trial utilizing AAV2 yielded very low expression of AAT.98 A trial with AAV1 met with more success as sustained expression of AAT was achieved, but the levels were much lower than the therapeutic threshold.89 In a second trial with AAV1, levels of ~2% of the therapeutic level were sustained as long as 5 years.90,91
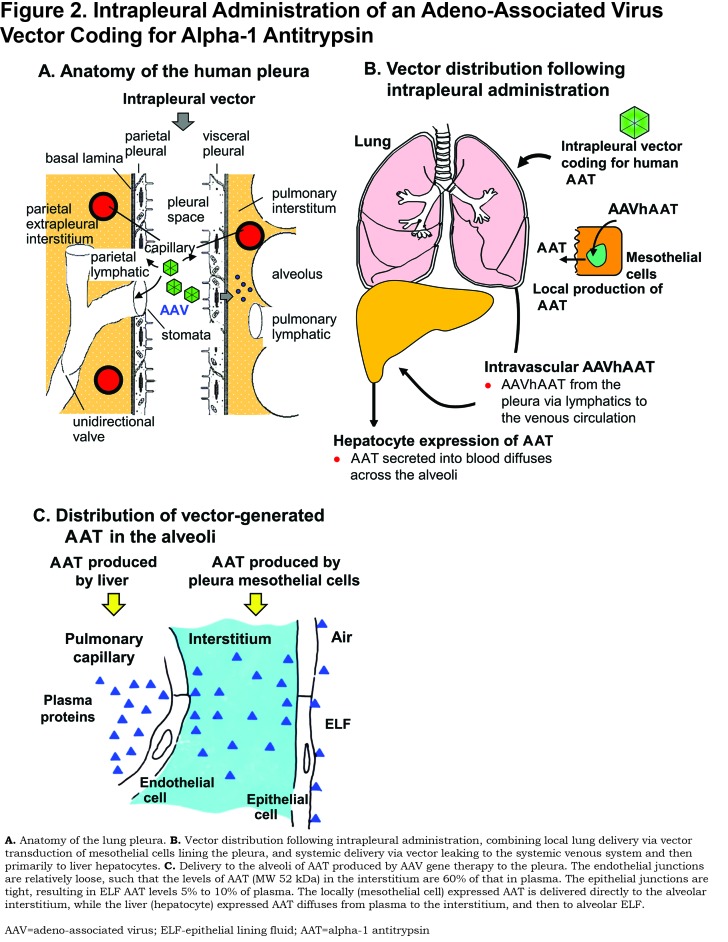
While each of these strategies target generally a single organ for vector transduction and AAT expression, intrapleural administration allows for both targeting of the lung directly and systemic delivery of the AAV gene transfer vector. The pleura is a thin serous membrane that encloses the chest cavity attaching the chest wall (parietal pleura) to the lung parenchyma (visceral pleura)99,100 (Figure 2A). Both the parietal and visceral pleura contain a single layer of mesothelial cells surrounded by a thin layer of connective tissue rich in lymphatic and blood vessels that are connected to the systemic circulation. The pleura layers are separated by a pleural fluid (0.5 to 1 ml in humans).100-105 Intrapleural gene transfer vector delivery has the benefits of both local lung delivery by transduction of the mesothelial cells lining the pleura and systemic delivery from vector passing through open stomata in the visceral pleural lymphatics to the systemic circulation and then primarily to liver hepatocytes (Figure 2B). AAT produced by the mesothelial cells is secreted and diffuses into the lung parenchyma. Because the lymphatic system of the parietal pleura connects directly from the pleural space through stomata, this allows for parallel systemic distribution of the gene therapy vector to primarily the liver via the circulation.99,100,106 AAT produced in the liver can travel back to the lung through the circulatory system. The major advantage of intrapleural administration is that both the lung and liver are targeted by the gene therapy vector, increasing the possibility of producing a sufficient amount of AAT to provide a therapeutic effect (Figure 2C).
Preclinical Efficacy of Intrapleural Administration of AAV Vectors
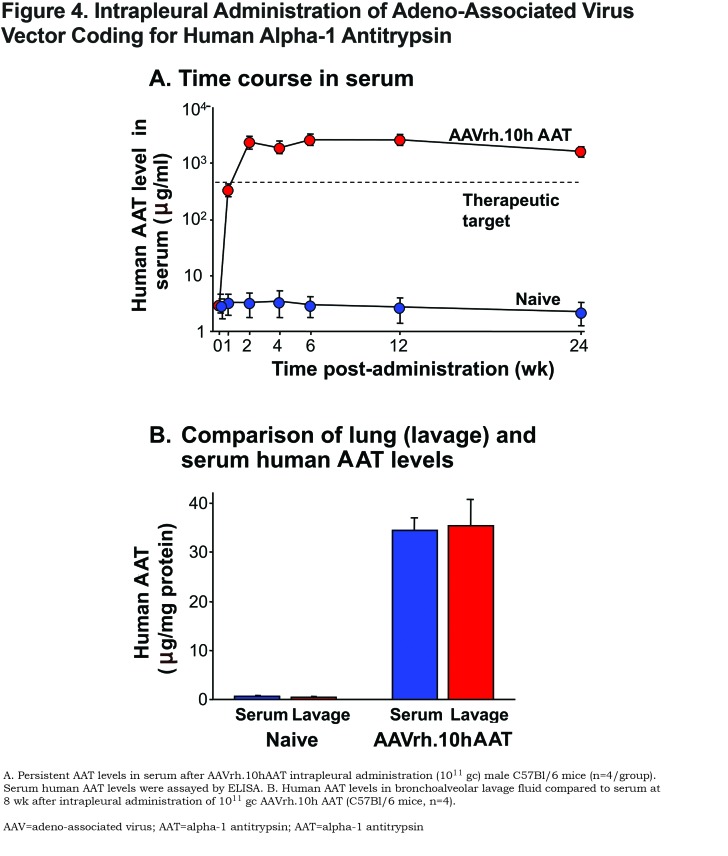
In a mouse preclinical model, De et al77 demonstrated that an AAV serotype 5-based vector expressing the human AAT gene produced higher levels of serum AAT via the intrapleural route of delivery compared to intramuscular administration at the same dose. Moreover, the AAV5-based vector produced about 8-fold higher levels of AAT compared to the levels achieved by an AAV2-based vector via both the intramuscular and intrapleural route. Intrapleural delivery of AAV5 coding for human AAT (administered dose of 1011 gc) mediated AAT serum levels of 900 ±50 μg/ml that were sustained up to 40 weeks post-administration. This level is significantly greater (1.6-fold) than the therapeutic threshold level of 570 μg/ml (11 μM). The AAT levels in the bronchoalveolar lavage fluid were similar to that in serum, indicating local production of AAT in the lungs.77 These observations lend support to the concept that AAV5-mediated intrapleural delivery of the AAT transgene can provide sufficient amounts of AAT to be able to protect the lung from proteolytic damage.
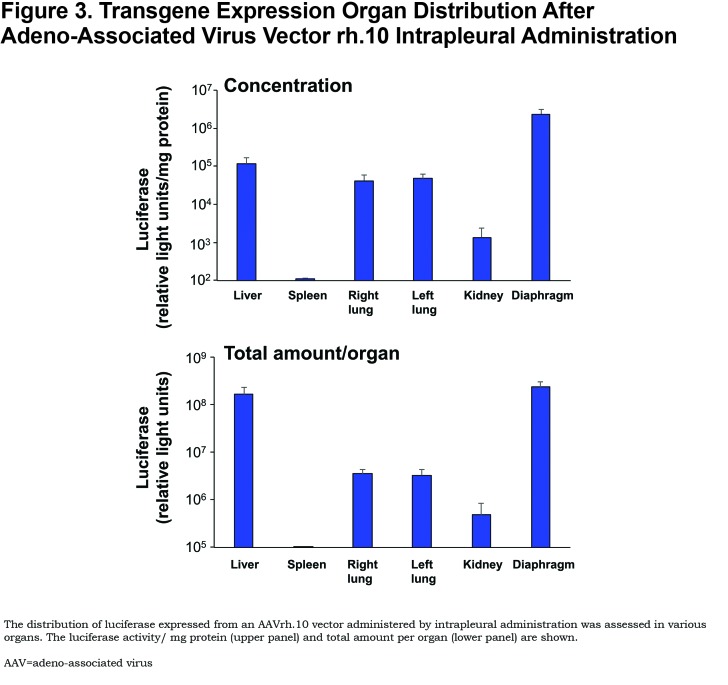
To identify a more potent AAV serotype than AAV5, De et al78 compared 25 different AAV serotypes (16 NHPs and 9 human AAV serotypes; all using the AAV2 inverted terminal repeats flanking the same human AAT cDNA driven by the CAG promoter) in mice (Figure 1). The authors demonstrated that intrapleural administration of the AAV nonhuman primate derived serotypes AAVrh.10 and AAV8 (both clade E) were the most effective at providing high serum AAT levels. Of the other serotypes tested, 11 were derived from rhesus macaques (AAV7, AAVrh.2, AAVrh.8, AAVrh.13, AAVrh.16, AAVrh.20, AAVrh.21, AAVrh.22, AAVrh.24, AAVrh.34 and AAVrh.43), and 1 was derived from cynomolgus macaque (AAVcy.5), 1 from baboon (AAVbb.2), 1 from chimpanzee (AAVch.5); and 9 from humans (AAV2, AAV5, AAV9, AAVhu.1, AAVhu.11, AAVhu.13, AAVhu.37, AAVhu.41, and AAVhu.47). The AAVrh.10 vector was chosen for further study. Administration of the AAVrh.10 vector (1011 gc) via the intrapleural route in the left lung resulted in high levels of transgene expression in the lungs, diaphragm, and liver (Figure 3). Additionally, this route of administration with AAVrh.10 produced sustained therapeutic levels of serum AAT (>2.5-fold above the minimum of 570 μg/ml, compared to the 1.6-fold levels produced by AAV5) up to 24 weeks, the latest point of the study (Figure 4A). The high serum AAT levels translated to similar therapeutic levels in lung ELF, demonstrating biochemical efficacy (Figure 4B). Importantly, the AAVrh.10 vector-produced AAT was functional in the inhibition of neutrophil elastase. In the context that AAV vectors are less potent in female mice, administration of AAVrh.10 vector (1011 gc) via the intrapleural route produced greater than therapeutic levels of AAT not just in male mice but also in female mice. The AAVrh.10 vector is derived from rhesus macaque, and therefore, another potential advantage is that pre-existing anti-vector immunity in humans is minimal.107 To assess whether the AAVrh.10 vector is functional in the presence of pre-existing immunity against the common human serotypes AAV2 and AAV5, administration of the AAVrh.10hAAT to AAV2- and AAV5-preimmune mice showed high level expression of AAT compared to nonimmune mice, thus demonstrating that AAVrh.10 is capable of circumventing common human immunity to AAV.78
AAVrh.10hAAT Safety and Toxicology Study
Based on the promising efficacy data with AAVrh.10hAAT in mice, the novel intrapleural delivery approach advanced to a safety and toxicology study.74 This study included 280 mice and 36 NHPs. The AAVrh.10 vector was administered via the intrapleural route at 2 doses in each species (1010 and 1011 gc in mice, 1012 and 1013 gc in NHPs). The safety of the intrapleural vector delivery assessment parameters included hematology, serum chemistry and histopathology. Additionally, vector genome biodistribution and transgene expression were evaluated at multiple time points over 6 months.
The mouse toxicology study involved 2 parts. For the primary study, 120 male and 120 female mice were administered either PBS or AAVrh.10hAAT (1010 gc or 1011 gc) by the intrapleural route, with assessment of: (1) safety following vector intrapleural administration; (2) biodistribution of the vector; and (3) hAAT mRNA expression in chest cavity organs over a course of 6 months. The second study assessed the potential toxicity of direct injection of PBS (control) or 1011 gc AAVrh.10hAAT into lung parenchyma in 20 male and 20 female mice, as a worse case scenario model for misplaced dosing during intrapleural administration over the course of a month. Overall, the AAVrh.10hAAT vector was well tolerated without any vector-related morbidity or mortality in either group, except a few surgical procedure-related deaths in the group receiving vector via intrapleural administration (n=8 of 240). For both studies, the assessment of hematology, serum chemistry and histopathology showed the therapy to be safe. In the intrapleural study, all of the vector-administered groups of mice developed dose-dependent AAVrh.10 neutralizing antibodies. Assessment of vector DNA in various organs showed high levels of transduction of the liver (>106 copies/μg total DNA at the high dose and >104 copies/μg total DNA at the low dose) followed by the diaphragm and lungs, while other organs had low vector DNA levels, detectable only in the higher dose group. To assess whether intrapleural delivery of the vector mediated sufficient transgene expression in the chest cavity, AAT mRNA levels were quantified in chest cavity organs. The diaphragm had the highest levels of mRNA (>106 copies/μg total RNA at the high dose and >104 copies/μg total RNA at the low dose), and these levels were sustained up to 182 days, the latest time point of the study. Similar high levels of AAT mRNA were detectable in other chest cavity organs including pleura, left lung and right lung, demonstrating efficient transgene delivery to the proximity of the lung and hence availability to protect the lung from proteolytic damage.
The NHP study included 18 male and 18 female African green monkeys. Overall safety and vector-mediated expression of AAT in the chest cavity was assessed. All animals remained healthy with normal weight gain, heart rate and respiratory rate. Overall hematology parameters and serum chemistry data were normal, with only a few sporadic changes in individual animals that were not statistically significant. Pathology and organ weights measured at necropsy showed that there were no vector-related gross anatomic changes and organ-to-body and organ-to-brain weights remained normal. No vector-related significant histopathological changes were observed. Assessment of AAVrh.10 neutralizing antibody levels in serum showed dose-dependent AAVrh.10 neutralizing antibody titers, persisting up to 360 days, the latest time point of the study. Finally, quantification of human AAT mRNA in the chest cavity tissues demonstrated high levels of human AAT mRNA localized to the proximity of the lung with the mRNA levels persisting up to 360 days at >104 copies/μg total RNA in chest wall pleura, diaphragm, and diaphragm pleura.
The combined data from these pivotal toxicology studies in 2 species (mice and NHPs) demonstrated that the approach of delivering AAVrh.10hAAT by the intrapleural route is both efficient and safe with no toxicity issues. This study provided the groundwork for the planned initiation of a clinical trial of intrapleural human AAVrh.10hAAT for the treatment of AAT deficiency.
Design of the Clinical Trial
On the basis of the above described efficacy and safety/toxicology studies of intrapleural administration of a serotype rh.10 replication-deficient AAV gene transfer vector expressing the human AAT cDNA in mice and in NHPs, an investigational new drug (IND) application was submitted to the FDA. This IND has been granted by the FDA (BBIND 16008) and has been licensed to Adverum Biotechnologies. The allowed clinical study (Protocol No. 1401014659, Clinical Trial ID: NCT02168686) is a gene transfer strategy designed to provide persistent high levels of human AAT. The study design has been previously published108 and is summarized here.
This study is designed as a phase I/II clinical trial to assess the safety and determine the preliminary efficacy of AAVrh.10hAAT (expressing the normal M1-type AAT) in humans with AATD. It is a 2-dose, open label study with n=5 individuals in each dose cohort (8x1012 and 8 x1013 gc) receiving the vector by the intrapleural delivery route. In addition, as a comparison to the intrapleural route, n=5 AAT-deficient individuals at each dose level will instead be administered the AAVrh.10h AAT vector by the intravenous route. A total of n=20 individuals with a genotype of ZZ or Z Null, with serum AAT levels of <11μM will be recruited to participate in this study. All participants will be monitored before and after vector administration with a variety of safety measures. In addition, biologic efficacy parameters will be assessed to generate preliminary assessment of the therapeutic impact of this intervention including serum and ELF AAT levels and function. The goal for the treatment to be considered efficacious and therapeutic is for the serum AAT levels to be >11 μM and AAT levels in the lung ELF to be >1.2 μM, the levels considered to be the "protective level" for AAT.63
Intrapleural AAT Gene Therapy - Moving Forward
Information gleaned from previous AAT clinical trials combined with the data from the preclinical studies suggest that intrapleural delivery of an AAVrh.10hAAT is a promising treatment for AATD. The upcoming clinical trials will help to determine whether administration of this vector by the intrapleural route will finally allow gene therapy to achieve the target protective level for biochemical efficacy. Achieving this primary end goal still opens the door for further questions to be evaluated. The next step after a demonstration of biochemical efficacy would be to evaluate clinical efficacy by monitoring stabilization of lung function or employing computer tomography lung density scans at total lung capacity to evaluate the rate of progression of lung destruction, as recently shown for AAT protein supplementation therapy.64,65 The advantage of AAT gene therapy over the current protein supplementation therapy is the potential for a single administration of the treatment. AAV vectors have been shown to drive persistent AAT expression out to at least 5 years with little diminution in the level of expression.90,91 However, the total longevity of expression is not yet known, and this leaves the possibility that readministration of the AAT gene therapy may be necessary during the lifetime of the patient. Future studies would be needed to help determine if an alteration in AAV serotype or the use of an immunodepressant regimen might be required for readministration or whether additional enhancements to current vectors might further improve gene expression stability.
Abbreviations
alpha-1 antitrypsin deficiency, AATD; alpha-1 antitrypsin, AAT; adeno-associated virus, AAV; serine protease inhibitor, SERPIN; epithelial lining fluid, ELF; Food and Drug Administration, FDA; nonhuman primate, NHP; investigational new drug, IND
References
- 1.Blanco I,Bueno P,Diego I,et al. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide:an update. Int J Chron Obstruct Pulmon Dis. 2017;12:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Serres FJ,Blanco I. Prevalence of alpha1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ:a comprehensive review. Ther Adv Respir Dis. 2012;6:277-295. doi: https://doi.org/10.1177/1753465812457113 [DOI] [PubMed] [Google Scholar]
- 3.Luisetti M,Seersholm N. Alpha1-antitrypsin deficiency. 1:epidemiology of alpha1-antitrypsin deficiency. Thorax. 2004;59(2):164-169. doi: https://doi.org/10.1136/thorax.2003.006494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoller JK,Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):246-259. doi: https://doi.org/10.1164/rccm.201108-1428CI [DOI] [PubMed] [Google Scholar]
- 5.Brantly M,Nukiwa T,Crystal RG. ) Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84(Supp6):13-31. doi: https://doi.org/10.1016/S0002-9343(88)80066-4 [DOI] [PubMed] [Google Scholar]
- 6.Brantly ML,Paul LD,Miller BH,et al. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms-1988. Am Rev Respir Dis. 138(2):327-336. doi: https://doi.org/10.1164/ajrccm/138.2.327 [DOI] [PubMed] [Google Scholar]
- 7.Crystal RG. Alpha 1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J Clin Invest. 1990;85:1343-1352. doi: https://doi.org/10.1172/JCI114578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lomas DA,Parfrey H. Alpha1-antitrypsin deficiency. 4:Molecular pathophysiology. Thorax. 2004;59(6):529-535. doi: https://doi.org/10.1136/thx.2003.006528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanash HA,Nilsson PM,Nilsson JA,Piitulainen E. Survival in severe alpha-1-antitrypsin deficiency (PiZZ). Respir Res. 2010;11:44. doi: https://doi.org/10.1186/1465-9921-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuder RM,Janciauskiene SM,Petrache I. Lung disease associated with alpha1-antitrypsin deficiency. Proc Am Thorac Soc. 2010;7(6):381-386. doi: https://doi.org/10.1513/pats.201002-020AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Alpha-1-Antitrypsin Deficiency Registry Study Group; Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med. 1998;158(1):49-59. [DOI] [PubMed] [Google Scholar]
- 12.Alam S,Li Z,Janciauskiene S,Mahadeva R. Oxidation of Z alpha1-antitrypsin by cigarette smoke induces polymerization:a novel mechanism of early-onset emphysema. Am J Respir Cell Mol Biol. 2011;45(2):261-269. doi: https://doi.org/10.1165/rcmb.2010-0328OC [DOI] [PubMed] [Google Scholar]
- 13.Crystal RG,Brantly ML,Hubbard RC,Curiel DT,States DJ,Holmes MD. The alpha 1-antitrypsin gene and its mutations. Clinical consequences and strategies for therapy. Chest. 1989;95(1):196-208. doi: https://doi.org/10.1378/chest.95.1.196 [DOI] [PubMed] [Google Scholar]
- 14.Lockett AD,Van Demark M,Gu Y,et al. Effect of cigarette smoke exposure and structural modifications on the alpha-1 antitrypsin interaction with caspases. Mol Med. 2012;18:445-454. doi: https://doi.org/10.2119/molmed.2011.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberici F,Martorana D,Vaglio A. (2014) Genetic aspects of anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2014;30(Suppl 1):i37-i45. doi: https://doi.org/10.1093/ndt/gfu386 [DOI] [PubMed] [Google Scholar]
- 16.de Serres F,Blanco I. Role of alpha-1 antitrypsin in human health and disease. J Intern Med. 2014;276(4):311-335. doi: https://doi.org/10.1111/joim.12239 [DOI] [PubMed] [Google Scholar]
- 17.Duvoix A,Roussel BD,Lomas DA. Molecular pathogenesis of alpha-1-antitrypsin deficiency. Rev Mal Respir. 2014;31(10):992-1002. doi: https://doi.org/10.1016/j.rmr.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 18.Eden E,Mitchell D,Mehlman B,et al. Atopy, asthma, and emphysema in patients with severe alpha-1-antitrypysin deficiency. Am J Respir Crit Care Med. 1997;156(1):68-74. doi: https://doi.org/10.1164/ajrccm.156.1.9508014 [DOI] [PubMed] [Google Scholar]
- 19.Inaty H,Arabelovic S. Alpha1-antitrypsin deficiency in a patient diagnosed with granulomatosis with polyangiitis. BMJ Case Rep. 2013. doi: https://doi.org/10.1136/bcr-2013-009045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perlmutter DH,Silverman GA. Hepatic fibrosis and carcinogenesis in alpha1-antitrypsin deficiency:a prototype for chronic tissue damage in gain-of-function disorders. Cold Spring Harb Perspect Biol 3. 2011;3(3):a005801. doi: https://doi.org/10.1101/cshperspect.a005801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone H,Pye A,Stockley RA. Disease associations in alpha-1-antitrypsin deficiency. Respir Med. 2014;108(2):338-343. doi: https://doi.org/10.1016/j.rmed.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 22.Teckman JH. Liver disease in alpha-1 antitrypsin deficiency:current understanding and future therapy. COPD. 2013;10(Suppl1):35-43. doi: https://doi.org/10.3109/15412555.2013.765839 [DOI] [PubMed] [Google Scholar]
- 23.Topic A,Radojkovic D. Polymerization and oxidation of alpha-1-antitrypsin in pathogenesis of emphysema. In:Irusen E, ed. Lung Diseases-Selected State of the Art Reviews. London: IntechOpenLimited;2012. http://www.intechopen.com/books/lung-diseases-selected-state-of-the-art-reviews/polymerization-and-oxidation-of-alpha-1-antitrypsin-in-pathogenesis-of-emphysema Accessed January 2018. [Google Scholar]
- 24.Guyot N,Wartelle J,Malleret L,et al. Unopposed cathepsin G, neutrophil elastase, and proteinase 3 cause severe lung damage and emphysema. Am J Pathol. 2014;184(8):2197-2100. doi: https://doi.org/10.1016/j.ajpath.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 25.Bergin DA,Reeves EP,Hurley K,et al. The circulating proteinase inhibitor alpha-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci Transl Med. 2014;6(217)doi: https://doi.org/10.1126/scitranslmed.3007116 [DOI] [PubMed] [Google Scholar]
- 26.Duranton J,Bieth JG. (2003) Inhibition of proteinase 3 by [alpha]1-antitrypsin in vitro predicts very fast inhibition in vivo. Am J Respir Cell Mol Biol. 2003;29(1):57-61. doi: https://doi.org/10.1165/rcmb.2002-0258OC [DOI] [PubMed] [Google Scholar]
- 27.Geraghty P,Eden E,Pillai M,Campos M,McElvaney NG,Foronjy RF. Alpha1-antitrypsin activates protein phosphatase 2A to counter lung inflammatory responses. Am J Respir Crit Care Med. 2014;190(11):1229-1242. doi: https://doi.org/10.1164/rccm.201405-0872OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janciauskiene SM,Nita IM,Stevens T. Alpha1-antitrypsin, old dog, new tricks. Alpha1-antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem. 2007;282:8573-8582. doi: https://doi.org/10.1074/jbc.M607976200 [DOI] [PubMed] [Google Scholar]
- 29.Jonigk D,Al-Omari M,Maegel L,et al. Anti-inflammatory and immunomodulatory properties of alpha1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci U S A. 2013;110(37):15007-15012. doi: https://doi.org/10.1073/pnas.1309648110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaner Z,Ochayon DE,Shahaf G,et al. Acute phase protein alpha1-antitrypsin reduces the bacterial burden in mice by selective modulation of innate cell responses. J Infect Dis. 2015;211(9):1489-1498. doi: https://doi.org/10.1093/infdis/jiu620 [DOI] [PubMed] [Google Scholar]
- 31.Lewis EC. Expanding the clinical indications for alpha(1)-antitrypsin therapy. Mol Med. 2012;18:957-970. doi: https://doi.org/10.2119/molmed.2011.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrache I,Fijalkowska I,Medler TR,et al. A-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169(4):1155-1166. doi: https://doi.org/10.2353/ajpath.2006.060058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahaf G,Moser H,Ozeri E,Mizrahi M,Abecassis A,Lewis EC. Alpha-1-antitrypsin gene delivery reduces inflammation, increases T-regulatory cell population size and prevents isletallograft rejection. Mol Med. 2011;17:1000-1011. doi: https://doi.org/10.2119/molmed.2011.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer LT,Paone G,Krein PM,Rouhani FN,Rivera-Nieves J,Brantly ML. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am J Physiol Lung Cell Mol Physiol. 2004;286:L514-L520. doi: https://doi.org/10.1152/ajplung.00099.2003 [DOI] [PubMed] [Google Scholar]
- 35.Sinden NJ,Baker MJ,Smith DJ,Kreft JU,Dafforn TR,Stockley RA. Alpha-1-antitrypsin variants and the proteinase/antiproteinase imbalance in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2015;308:L179-L190. doi: https://doi.org/10.1152/ajplung.00179.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadek JE,Fells GA,Zimmerman RL,Rennard SI,Crystal RG. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981;68:889-898. doi: https://doi.org/10.1172/JCI110344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene CM,Miller SD,Carroll T,et al. Alpha-1 antitrypsin deficiency:a conformational disease associated with lung and liver manifestations. J Inherit Metab Dis. 2008;31(1):21-34. doi: https://doi.org/10.1007/s10545-007-0748-y [DOI] [PubMed] [Google Scholar]
- 38.Silverman EK,Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl JMed. 2009;360:2749-2757. doi: https://doi.org/10.1056/NEJMcp0900449 [DOI] [PubMed] [Google Scholar]
- 39.Cichy J,Potempa J,Travis J. Biosynthesis of alpha1-proteinase inhibitor by human lung-derived epithelial cells. J Biol Chem. 1997;272(13):8250-8255. doi: https://doi.org/10.1074/jbc.272.13.8250 [DOI] [PubMed] [Google Scholar]
- 40.du Bois RM,Bernaudin JF,Paakko P,et al. Human neutrophils express the alpha 1-antitrypsin gene and produce alpha 1-antitrypsin. Blood. 1991;77:2724-2730. [PubMed] [Google Scholar]
- 41.Mornex JF,Chytil-Weir A,Martinet Y,Courtney M,LeCocq JP,Crystal RG. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986;77:1952-1961. doi: https://doi.org/10.1172/JCI112524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van 't Wout EF,van Schadewijk A,Savage ND,Stolk J,Hiemstra PS. Alpha1-antitrypsin production by proinflammatory and antiinflammatory macrophages and dendritic cells. Am J Respir Cell Mol Biol. 2012;46:365-376. [DOI] [PubMed] [Google Scholar]
- 43.Venembre P,Boutten A,Seta N,et al. Secretion of alpha 1-antitrypsin by alveolar epithelial cells. FEBS Lett. 1994;346:171-174. doi: https://doi.org/10.1074/jbc.272.13.8250 [DOI] [PubMed] [Google Scholar]
- 44.Crystal RG. The alpha 1-antitrypsin gene and its deficiency states. Trends Genet. 1989;5:411-417. doi: https://doi.org/10.1016/0168-9525(89)90200-X [DOI] [PubMed] [Google Scholar]
- 45.Loring HS,Flotte TR. Current status of gene therapy for alpha-1 antitrypsin deficiency. Expert Opin Biol Ther. 2015;15(3):329-336. doi: https://doi.org/10.1517/14712598.2015.978854 [DOI] [PubMed] [Google Scholar]
- 46.Cox DW,Markovic VD,Teshima IE. Genes for immunoglobulin heavy chains and for alpha 1-antitrypsin are localized to specific regions of chromosome 14q. Nature. 1982;297:428-430. doi: https://doi.org/10.1038/297428a0 [DOI] [PubMed] [Google Scholar]
- 47.Laurell C-B,Eriksson S. The electrophoretic alpha1-globulin pattern of serum in alpha1-antitrypsin deficiency. Scand J Clin Lab Invest. 1963;15(2):132-140. doi: https://doi.org/10.1080/00365516309051324 [Google Scholar]
- 48.Schroeder WT,Miller MF,Woo SL,Saunders GF. Chromosomal localization of the human alpha 1-antitrypsin gene (PI) to 14q31-32. Am J Hum Genet. 1985;37(5):868-872. [PMC free article] [PubMed] [Google Scholar]
- 49.Seixas S,Garcia O,Trovoada MJ,Santos MT,Amorim A,Rocha J. Patterns of haplotype diversity within the serpin gene cluster at 14q32. 1:insights into the natural history of the alpha1-antitrypsin polymorphism. Hum Genet. 2001;108(1):20-30. doi: https://doi.org/10.1007/s004390000434 [DOI] [PubMed] [Google Scholar]
- 50.Kurachi K,Chandra T,Degen SJ,et al. Cloning and sequence of cDNA coding for alpha 1-antitrypsin. Proc Natl Acad Sci U S A. 1981;78:6826-6830. doi: https://doi.org/10.1073/pnas.78.11.6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birrer P,McElvaney NG,Chang-Stroman LM,Crystal RG. Alpha 1-antitrypsin deficiency and liver disease. J Inherit Metab Dis. 1991;14(4):512-525. doi: https://doi.org/10.1007/BF01797921 [DOI] [PubMed] [Google Scholar]
- 52.Gooptu B,Lomas DA. Conformational pathology of the serpins:themes, variations, and therapeutic strategies. Annu Rev Biochem. 2009;78:147-176. doi: https://doi.org/10.1146/annurev.biochem.78.082107.133320 [DOI] [PubMed] [Google Scholar]
- 53.Jeppsson JO. (1976) Amino acid substitution Glu leads to Lys alpha1-antitrypsin PiZ. FEBSLett. 1976;65(2):195-197. doi: https://doi.org/10.1016/0014-5793(76)80478-4 [DOI] [PubMed] [Google Scholar]
- 54.Lomas DA,Evans DL,Finch JT,Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605-607. doi: https://doi.org/10.1038/357605a0 [DOI] [PubMed] [Google Scholar]
- 55.Bornhorst JA,Greene DN,Ashwood ER,Grenache DG. Alpha1-Antitrypsin phenotypes and associated serum protein concentrations in a large clinical population. Chest. 2013;143(4):1000-1008. doi: https://doi.org/10.1378/chest.12-0564 [DOI] [PubMed] [Google Scholar]
- 56.Long GL,Chandra T,Woo SL,Davie EW,Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984;23(21):4828-4837. doi: https://doi.org/10.1021/bi00316a003 [DOI] [PubMed] [Google Scholar]
- 57.Ogushi F,Hubbard RC,Fells GA,et al. Evaluation of the S-type of alpha-1-antitrypsin as an in vivo and in vitro inhibitor of neutrophil elastase. Am Rev Respir Dis. 1988;137(2):364-370. doi: https://doi.org/10.1164/ajrccm/137.2.364 [DOI] [PubMed] [Google Scholar]
- 58.Owen MC,Carrell RW,Brennan SO. The abnormality of the S variant of human alpha-1-antitrypsin. Biochim Biophys Acta. 1976;453:257-261. doi: https://doi.org/10.1016/0005-2795(76)90271-3 [DOI] [PubMed] [Google Scholar]
- 59.Yoshida A,Ewing C,Wessels M,Lieberman J,Gaidulis L. Molecular abnormality of PI S variant of human alpha1-antitrypsin. Am J Hum Genet. 1977;29:233-239. [PMC free article] [PubMed] [Google Scholar]
- 60.Sandford AJ,Weir TD,Spinelli JJ,Pare PD. Z and S mutations of the alpha1-antitrypsin gene and the risk of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 1999;20(2):287-291. doi: https://doi.org/10.1165/ajrcmb.20.2.3177 [DOI] [PubMed] [Google Scholar]
- 61.Chiuchiolo MJ,Crystal RG. Gene therapy for alpha-1 antitrypsin deficiency lung disease. Ann Am Thorac Soc. 2016;13(Suppl4):S352-S369. doi: https://doi.org/10.1513/AnnalsATS.201506-344KV [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gadek JE,Klein HG,Holland PV,Crystal RG. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981;68:1158-1165. doi: https://doi.org/10.1172/JCI110360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wewers MD,Casolaro MA,Sellers SE,et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316:055-1062. doi: https://doi.org/10.1056/NEJM198704233161704 [DOI] [PubMed] [Google Scholar]
- 64.Chapman KR,Burdon JG,Piitulainen E,et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID):a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360-368. doi: https://doi.org/10.1016/S0140-6736(15)60860-1 [DOI] [PubMed] [Google Scholar]
- 65.McElvaney NG,Burdon J,Holmes M,Holmes M. (for the Rapid Extension Trial Group). Long-term efficacy and safety of alpha1 proteinase inhibitor treatment for emphysema caused by severe alpha1 antitrypsin deficiency:an open-label extension trial (RAPID-OLE). LancetRespir Med. 2017;5:51-60. [DOI] [PubMed] [Google Scholar]
- 66.American Thoracic Society/European Respiratory Society statement:standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency; . Am J Respir Crit Care Med. 2003;168(7):818-900. doi: https://doi.org/10.1164/rccm.168.7.818 [DOI] [PubMed] [Google Scholar]
- 67.Kaplan A,Cosentino L. Alpha1-antitrypsin deficiency:forgotten etiology. Can Fam Physician. 2010;56:19-24. [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson Healthcare; Red Book:pharmacy's fundamental reference. Thomson Reuters. :NewYork,NY;2010. [Google Scholar]
- 69.Sondhi D,Stiles KM,De BP,Crystal RG. Genetic modification of the lung directed toward treatment of human disease. Hum Gene Ther. 2017;28(1):3-84. doi: https://doi.org/10.1089/hum.2016.152 [DOI] [PubMed] [Google Scholar]
- 70.Daya S,Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manno CS,Pierce GF,Arruda VR,et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342-347. doi: https://doi.org/10.1038/nm1358 [DOI] [PubMed] [Google Scholar]
- 72.Gao G,Vandenberghe LH,Alvira MR,et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78(12):6381-6388. doi: https://doi.org/10.1128/JVI.78.12.6381-6388.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao G,Vandenberghe LH,Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5(3):285-297. doi: https://doi.org/10.2174/1566523054065057 [DOI] [PubMed] [Google Scholar]
- 74.Chiuchiolo MJ,Kaminsky SM,Sondhi D,et al. Intrapleural administration of an AAVrh.10 vector coding for human alpha1-antitrypsin for the treatment of alpha1-antitrypsin deficiency. Hum Gene Ther Clin Dev. 2013;24(4):161-173. doi: https://doi.org/10.1089/humc.2013.168 [DOI] [PubMed] [Google Scholar]
- 75.Chulay JD,Ye GJ,Thomas DL,et al. Preclinical evaluation of a recombinant adeno-associated virus vector expressing human alpha-1 antitrypsin made using a recombinant herpes simplex virus production method. Hum Gene Ther. 2011;22(2):155-165. doi: https://doi.org/10.1089/hum.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conlon TJ,Cossette T,Erger K,et al. Efficient hepatic delivery and expression from a recombinant adeno-associated virus 8 pseudotyped alpha1-antitrypsin vector. Mol Ther. 2005;12(5):867-875. doi: https://doi.org/10.1016/j.ymthe.2005.05.016 [DOI] [PubMed] [Google Scholar]
- 77.De B,Heguy A,Leopold PL,et al. Intrapleural administration of a serotype 5 adeno-associated virus coding for alpha1-antitrypsin mediates persistent, high lung and serum levels of alpha1-antitrypsin. Mol Ther. 2004;10(6):1003-1010. doi: https://doi.org/10.1016/j.ymthe.2004.08.022 [DOI] [PubMed] [Google Scholar]
- 78.De BP,Heguy A,Hackett NR,et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther. 2006;13(1):67-76. doi: https://doi.org/10.1016/j.ymthe.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 79.Flotte TR,Conlon TJ,Poirier A,Campbell-Thompson M,Byrne BJ. Preclinical characterization of a recombinant adeno-associated virus type 1-pseudotyped vector demonstrates dose-dependent injection site inflammation and dissemination of vector genomes to distant sites. Hum Gene Ther. 2007;18(3):245-256. doi: https://doi.org/10.1089/hum.2006.113 [DOI] [PubMed] [Google Scholar]
- 80.Halbert CL,Madtes DK,Vaughan AE,et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172. doi: https://doi.org/10.1038/mt.2010.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Limberis MP,Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci U S A. 2006;103(35):12993-12998. doi: https://doi.org/10.1073/pnas.0601433103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Limberis MP,Vandenberghe LH,Zhang L,Pickles RJ,Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther. 2009;17(2):294-301. doi: https://doi.org/10.1038/mt.2008.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liqun Wang R,McLaughlin T,Cossette T,et al. Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of alpha-1-antitrypsin using invasive and noninvasive delivery. Mol Ther. 2009;17(1):81-87. doi: https://doi.org/10.1038/mt.2008.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu Y,Choi YK,Campbell-Thompson M,et al. Therapeutic level of functional human alpha 1 antitrypsin (hAAT) secreted from murine muscle transduced by adeno-associated virus (rAAV1) vector. J Gene Med. 2006;8(6):730-735. doi: https://doi.org/10.1002/jgm.896 [DOI] [PubMed] [Google Scholar]
- 85.Song S,Embury J,Laipis PJ,Berns KI,Crawford JM,Flotte TR. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001;8:1299-1306. doi: https://doi.org/10.1038/sj.gt.3301422 [DOI] [PubMed] [Google Scholar]
- 86.Virella-Lowell I,Zusman B,Foust K,et al. Enhancing rAAV vector expression in the lung. J Gene Med. 2005;7(7):842-850. doi: https://doi.org/10.1002/jgm.759 [DOI] [PubMed] [Google Scholar]
- 87.Xiao W,Berta SC,Lu MM,Moscioni AD,Tazelaar J,Wilson JM. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222-10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu H,Buff SM,Baatz JE,Virella-Lowell I. Oral instillation with surfactant phospholipid:a reliable alternative to intratracheal injection in mouse studies. Lab Anim. 2008;42(3):294-304. doi: https://doi.org/10.1258/la.2007.007055 [DOI] [PubMed] [Google Scholar]
- 89.Brantly ML,Chulay JD,Wang L,et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106(38):16363-16368. doi: https://doi.org/10.1073/pnas.0904514106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flotte TR,Trapnell BC,Humphries M,et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin:interim results. Hum Gene Ther. 2011;22(10):1239-1247. doi: https://doi.org/10.1089/hum.2011.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mueller C,Gernoux G,Gruntman AM,et al. 5 Year expression and neutrophil D=defect repair after gene therapy in alpha-1 antitrypsin aeficiency. Mol Ther. 2017;25(6):1387-1394. doi: https://doi.org/10.1016/j.ymthe.2017.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duan D,Yue Y,Yan Z,McCray PB, Jr,Engelhardt JF. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9(18):2761-2776. doi: https://doi.org/10.1089/hum.1998.9.18-2761 [DOI] [PubMed] [Google Scholar]
- 93.Bals R,Xiao W,Sang N,Weiner DJ,Meegalla RL,Wilson JM. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J Virol. 1999;73:6085-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferrari S,Griesenbach U,Geddes DM,Alton E. Immunological hurdles to lung gene therapy. Clin Exp Immunol. 2003;132(1):1-8. doi: https://doi.org/10.1046/j.1365-2249.2003.02124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pickles RJ. Physical and biological barriers to viral vector-mediated delivery of genes to the airway epithelium. Proc Am Thorac Soc. 2004;1(4):302-308. doi: https://doi.org/10.1513/pats.200403-024MS [DOI] [PubMed] [Google Scholar]
- 96.Yang Y,Li Q,Ertl HC,Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song S,Morgan M,Ellis T,et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci U S A. 1998;95(24):14384-14388. doi: https://doi.org/10.1073/pnas.95.24.14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brantly ML,Spencer LT,Humphries M,et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alpha-1-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17(12):1177-1186. doi: https://doi.org/10.1089/hum.2006.17.1177 [DOI] [PubMed] [Google Scholar]
- 99.Finley DJ,Rusch VW. Anatomy of the pleura. Thorac Surg Clin. 2011;21(2):157-163. doi: https://doi.org/10.1016/j.thorsurg.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 100.Wang NS. Anatomy and physiology of the pleural space. Clin Chest Med. 6:3-16. [PubMed] [Google Scholar]
- 101.Agostoni E,Zocchi L. Pleural liquid and its exchanges. Respir Physiol Neurobiol. 2007;159(3):311-323. doi: https://doi.org/10.1016/j.resp.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 102.Mutsaers SE. Mesothelial cells:their structure, function and role in serosal repair. Respirology. 2002;7(3):171-191. doi: https://doi.org/10.1046/j.1440-1843.2002.00404.x [DOI] [PubMed] [Google Scholar]
- 103.Noppen M,De Waele M,Li R,et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med. 2000;162(3):1023-1026. doi: https://doi.org/10.1164/ajrccm.162.3.9910050 [DOI] [PubMed] [Google Scholar]
- 104.Noppen M. Normal volume and cellular contents of pleural fluid. Curr Opin Pulm Med. 2001;7(4):180-182. doi: https://doi.org/10.1097/00063198-200107000-00002 [DOI] [PubMed] [Google Scholar]
- 105.Rennard SI,Jaurand MC,Bignon J,et al. Role of pleural mesothelial cells in the production of the submesothelial connective tissue matrix of lung. Am Rev Respir Dis. 1984;130:267-274. [DOI] [PubMed] [Google Scholar]
- 106.Negrini D,Moriondo A. Pleural function and lymphatics. Acta Physiol (Oxf). 2013;207(2):244-259. doi: https://doi.org/10.1111/apha.12016 [DOI] [PubMed] [Google Scholar]
- 107.Boutin S,Monteilhet V,Veron P,et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population:implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704-712. doi: https://doi.org/10.1089/hum.2009.182 [DOI] [PubMed] [Google Scholar]
- 108.Chiuchiolo MJ,Kaminsky SM,Sondhi D,Mancenido D,Hollmann C,Crystal RG. Phase I/II study of intrapleural administration of a serotype rh.10 replication-deficient adeno-associated virus gene transfer vector expressing the human alpha1-antitrypsin cDNA to individuals with alpha1-antitrypsin deficiency. Hum Gene Ther Clin Dev. 2014;25(3):112-133. doi: https://doi.org/10.1089/humc.2014.2513 [DOI] [PubMed] [Google Scholar]