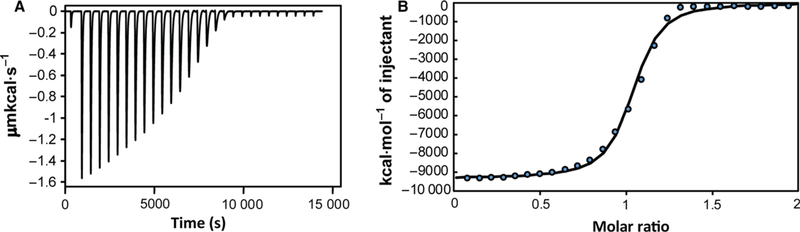
Fig. 2.
Titration of piscidin by Cu2+ in the presence of glycine as a competitor. (A) ITC data showing twenty-eight 10 μL-injections of 900 μM Cu2+ into 90 μM p1 performed in the presence of 5 mM glycine and 10 mM HEPES buffer (pH 7.4) at 25 °C. (B) Integrated titration data at increasing molar ratio of copper:piscidin showing the binding isotherm (with the individual experimental data points showing as blue circles) and the least-square fit of the data assuming a one binding-site model (solid line). This isotherm yielded an apparent binding constant between piscidin and Cu2+ that was used to obtain the corrected binding constant between p1 and Cu2+ at pH 7.4, as described in the text.