Figure 4. Schematic Diagrams of Allosteric PTM Examples.
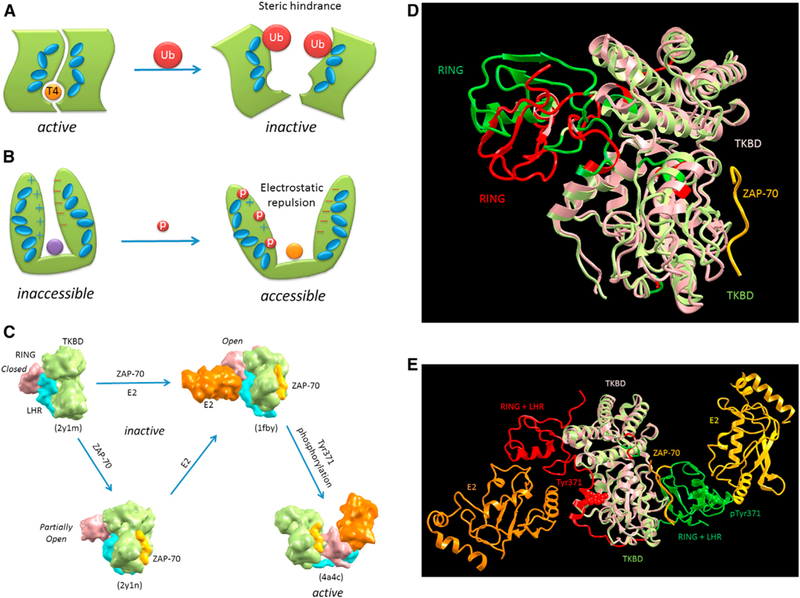
(A) illustratesan activethyroid hormone-activating type 2 deiodinasewith a bound T4 substrate. It istransiently inactivated by ubiquitylation at the dimer interface. The allosteric action of the ubiquitylations is illustrated by the interacting blue ellipsoids, which propagatethe steric hindrance down to the T4 binding site (Sagar et al., 2007). (B) Progressive phosphorylation events up to a certain threshold number at the N-terminal region of circadian clock protein, FRQ, allosterically expose its protected middle region (purple circle) and lead to its own degradation (Querfurth et al., 2011). The allosteric action is illustrated by the interacting blue ellipsoids, which propagate the created electrostatic repulsion between the N-terminal and C-terminal of FRQ down to the exposed middle region (orange circle). (C-E) A phosphorylation-dependent activation of c-Cbl, a RING ubiquitin ligase that attenuate receptortyrosine kinase signal transduction, is illustrated by four captured crystal structures with a combination of two involved allosteric actions. (C) provides a model for the binding of substrate sequences (the ZAP-70 peptide) to the N-terminal SH2-containing tyrosine kinase-binding domain (TKBD) and of ubiquitin-conjugating enzyme (E2) and Tyr371 phosphorylation, together leading toward c-Cbl activation (Dou et al., 2012). In the absence of E2 and the TKBD substrate, the c-CBl (a single-subunit RING E3 that negatively regulates proteins by promoting ubiquitination and subsequent degradation; PDB 2Y1M) adopts a closed conformation, in which the E2-binding surface of RING subdomain associates with the TKBD. The allosteric effect of the ZAP-70 peptide binding partially opens the RING subdomain, making it favorable for E2 binding. This is clearly seen from the superposition of the two crystal structures (PDB 2Y1M and 2Y1N) shown in (D), which indicate the partial RING opening (the RING movement from red to green) due to the TKBD substrate binding (yellow ribbon). The subsequent E2 binding causes the RING subdomain to adopt an open conformation. Tyr371, which mediates the linker helix region (LHR) subdomain binding to the TKBD, secures the c-Cbl in an inactive state. The phosphorylation ofTyr371 activates c-Cbl by releasing LHR from TKBD, which then undergoes a large conformational change that brings the RING subdomain and E2 into proximity of the substrate. The large conformational change due to the pTyr371 is seen from the superposition of two crystal structures (PDB 1FBV and 4A4C) in (E). Both the side chains of Tyr371 (red) and pTyr371 (green) are given in space-fill models. (A) and (B) are adapted with permission from Nussinov et al., (2012)
