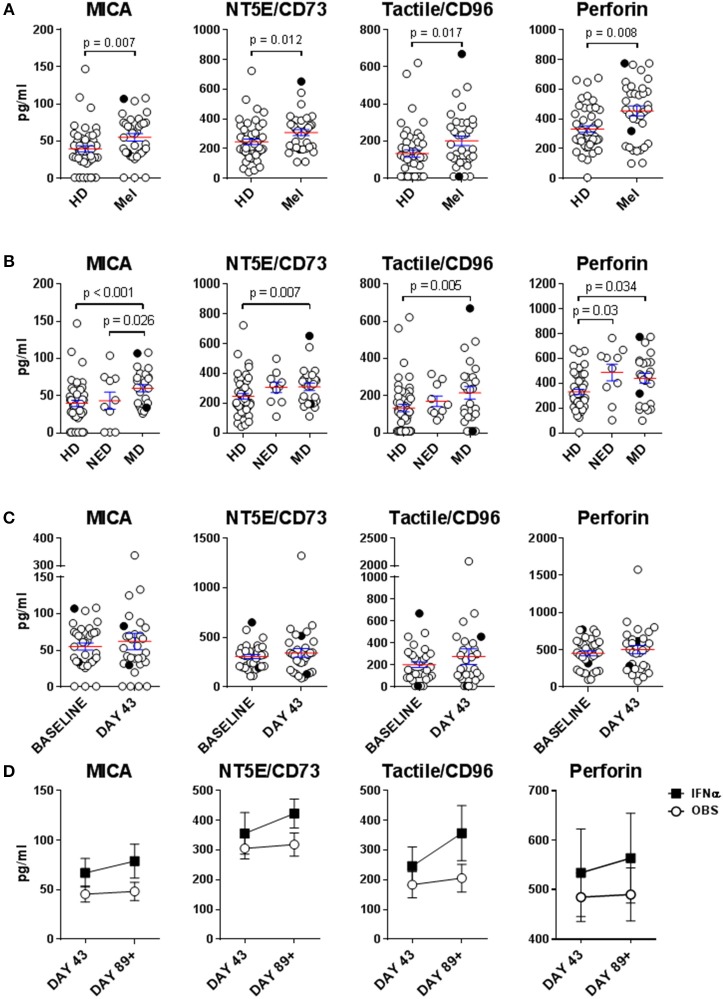
Figure 1.
Evaluation of immunosuppressive factors in donor sera. Presence of soluble immunosuppressive factors in sera isolated from healthy donors (HD) and melanoma patients (Mel) was measured by Luminex. (A,B) Mel sera isolated prior to treatments (Baseline) were evaluated (B) Patients with resected (NED) and unresectable (measurable disease; MD) tumors were compared for their serum concentrations of immunosuppressive factors to HDs. (C) Mel sera isolated prior to all the treatments (Baseline) and after DC immunizations (Day 43) are compared. (D) Mel sera isolated post DC immunizations are tested in patients in the observation arm of the study and those that received IFNα therapy (IFNα). Intersecting lines and whiskers represent mean and standard error of mean values, respectively. Black circles (A–C) represent patients that were PR.