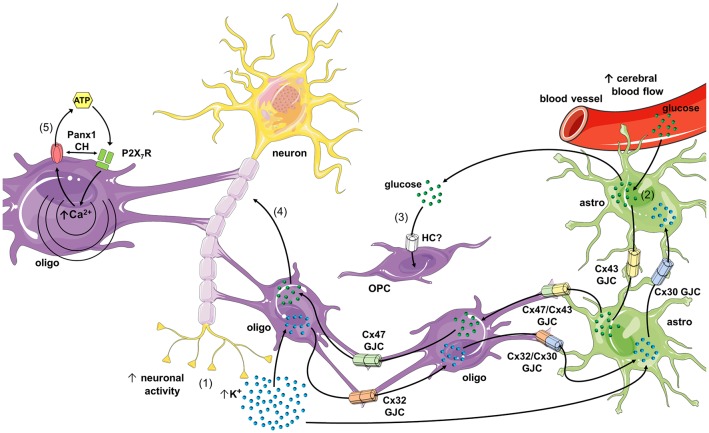
Figure 2.
Schematics of possible roles of connexin- and pannexin-based channels in oligodendrocyte function and dysfunction. During high rates of neuronal activity K+ accumulates in the extracellular space, and then is taken up by oligodendrocytes and astrocytes through the inwardly rectifying K+ channel (Kir) 4.1 and/or Na+/K+-pumps (1). K+ that concentrates inside oligodendrocytes and astrocytes diffuses to the panglial syncytium via homocellular and heterocellular gap junction channels (GJCs), a process termed “spatial K+ buffering.” In parallel, neuronal and astroglial signaling (e.g., ATP and glutamate) could activate endothelial P2 and NMDA receptors (NMDARs), respectively, leading to increased free [Ca2+]i and further vasodilation of blood vessels (not depicted). The latter increases cerebral blood flow and further uptake of glucose by astrocytic endfeet (2). Glucose diffuses through astrocytes and oligodendrocytes via homocellular and heterocellular GJCs and then can be metabolized to lactate by astrocytes, and both can be released into the extracellular space. In addition, glucose is taken up by oligodendrocyte precursor cells (OPCs; via an unknown hemichannel) and neurons, which possibly modulate oligodendrocyte differentiation and maturation (3), as well as axonal function (4), respectively. In pathological scenarios, the opening of Panx1 channels may lead to the release of ATP from oligodendrocytes (5), resulting in the activation of P2X7 receptors (P2X7Rs) and further triggering of a self-perpetuating mechanism of cell damage, in which high levels of [Ca2+]i and direct protein-protein interaction could reactivate pannexons. Part of this schematics was done with support of the free online Servier Medical Art repository (https://smart.servier.com/).