Abstract
Aortic stenosis is characterized both by progressive valve narrowing and the left ventricular remodeling response that ensues. The only effective treatment is aortic valve replacement, which is usually recommended in patients with severe stenosis and evidence of left ventricular decompensation. At present, left ventricular decompensation is most frequently identified by the development of typical symptoms or a marked reduction in left ventricular ejection fraction <50%. However, there is growing interest in using the assessment of myocardial fibrosis as an earlier and more objective marker of left ventricular decompensation, particularly in asymptomatic patients, where guidelines currently rely on nonrandomized data and expert consensus. Myocardial fibrosis has major functional consequences, is the key pathological process driving left ventricular decompensation, and can be divided into 2 categories. Replacement fibrosis is irreversible and identified using late gadolinium enhancement on cardiac magnetic resonance, while diffuse fibrosis occurs earlier, is potentially reversible, and can be quantified with cardiac magnetic resonance T1 mapping techniques. There is a substantial body of observational data in this field, but there is now a need for randomized clinical trials of myocardial imaging in aortic stenosis to optimize patient management. This review will discuss the role that myocardial fibrosis plays in aortic stenosis, how it can be imaged, and how these approaches might be used to track myocardial health and improve the timing of aortic valve replacement.
Key Words: aortic stenosis, cardiac magnetic resonance, late gadolinium enhancement, myocardial fibrosis, T1 mapping
Abbreviations and Acronyms: AVR, aortic valve replacement; CI, confidence interval; CMR, cardiac magnetic resonance; CT, computed tomography; ECV%, extracellular volume fraction; HR, hazard ratio; iECV, indexed extracellular volume; LGE, late gadolinium enhancement; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement
Central Illustration
Aortic stenosis is one of the most common valvular diseases in the Western world 1, 2, with an estimated prevalence as high as 12.4% in the elderly (3). Aortic stenosis is characterized not only by progressive valve obstruction, but also by the left ventricular remodeling response (4). Narrowing of the valve causes pressure overload of the left ventricle and triggers a hypertrophic response that maintains myocardial performance for many years, if not decades. However, with time, this process decompensates as patients transition from hypertrophy to heart failure, a change that is heralded clinically by the development of symptoms and adverse events, leading to consideration of aortic valve replacement (AVR).
Aortic stenosis progresses inexorably. Although the early stages are asymptomatic and associated with a good prognosis, advanced disease is associated with substantial morbidity and mortality 5, 6, 7. Despite much research, to date there are no proven medical therapies that slow disease progression. The only definitive treatment for severe aortic stenosis remains AVR, either by surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) approaches. The uptake of TAVR has grown exponentially 3, 8, as interventions that were initially offered only to elderly, inoperable patients are now being performed in younger, lower-risk patients with excellent results 9, 10, 11, 12, 13. Decisions about if, when, and how to intervene have therefore become increasingly complex, requiring careful assessment of individual patients within a multidisciplinary heart team.
Current guidelines recommend intervention in patients with severe aortic stenosis and evidence of left ventricular decompensation. Most commonly this is in the form of development of typical symptoms, but other markers include a reduction in ejection fraction <50%, an abnormal exercise tolerance test, or a rise in brain natriuretic peptide levels 14, 15. Unfortunately, symptoms are often difficult to identify in the elderly comorbid patients encountered in clinical practice, and many of the other changes appear only late in the course of the disease after irreversible myocardial damage has become established. European Society of Cardiology guidelines provide a Class 1 recommendation, Level of Evidence: B, for intervention in the most common scenario—symptomatic, severe aortic stenosis. However, intervention in asymptomatic patients with a reduction in ejection fraction <50% or an abnormal exercise test is only Level of Evidence: C (i.e., expert opinion) (15). The American College of Cardiology and American Heart Association guidelines are largely in alignment (14). This highlights the need for more robust data to better risk-stratify patients and optimize management strategies before the onset of symptoms and heart failure.
Consequently, there is extensive interest in identifying novel, objective markers of early left ventricular decompensation to optimize the timing of AVR and track myocardial health over time. The development of such markers requires improved understanding of the pathophysiology underling left ventricular decompensation in aortic stenosis. Histological studies have suggested that myocardial fibrosis and cell death are both important drivers of this process 16, 17. Attention has focused on myocardial fibrosis in particular, given its structure-function correlation with heart failure and the fact that it can now be identified reliably and noninvasively with modern imaging techniques. This review will discuss the pathophysiology of myocardial fibrosis and left ventricular decompensation in aortic stenosis, the imaging techniques that can be used to detect it, and how these might be employed to track myocardial health and optimize the timing of AVR.
Pathology
It is useful to consider aortic stenosis as a disease of both the valve and the myocardium (4). In addition, the importance of arterial stiffness and systemic pulsatile arterial load cannot be underestimated in this elderly population 18, 19. A detailed discussion of events within the valve is beyond the scope of this review (20); however, an understanding of the pathological factors driving the hypertrophic remodeling response and its subsequent decompensation are critical to understanding the rationale for myocardial fibrosis imaging (Central Illustration).
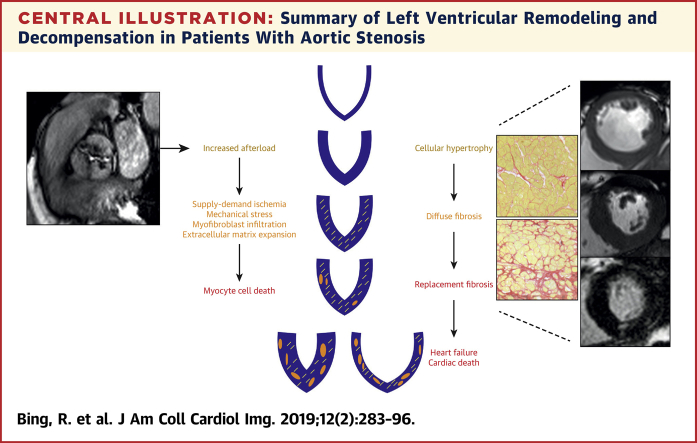
Central Illustration.
Summary of Left Ventricular Remodeling and Decompensation in Patients With Aortic Stenosis
Schematic of the left ventricular remodeling response in aortic stenosis, describing the transition from hypertrophy to fibrosis, heart failure, and cardiac death.
Progressive valve narrowing causes pressure overload of the left ventricle and triggers a hypertrophic response that maintains wall stress and left ventricular performance for many years. Over time, this process decompensates and patients transition from hypertrophy to heart failure, leading to adverse clinical outcomes. This evolution is complex but is closely related to the development of myocardial fibrosis, myocyte injury, and cell death. Furthermore, there is adverse remodeling of the extracellular matrix, with degradation and disruption of the matrix structure (21). These changes are regulated by several factors, including the renin-angiotensin-aldosterone system, transforming growth factor beta, apoptosis signal-regulating kinase 1, and tissue inhibitor of metalloproteinase 22, 23, 24: all potential targets for novel therapeutic interventions.
Two distinct myocardial fibrosis patterns have been described. Reactive interstitial fibrosis is diffuse and follows increased myofibroblast activity and collagen deposition that begins even in the early stages of aortic stenosis. Importantly, this diffuse fibrosis is reversible and has been demonstrated to regress following AVR (16). In contrast, replacement fibrosis appears to occur later and is irreversible (25). Treibel et al. (26) recently demonstrated that patients with advanced disease undergoing AVR manifest a complex combined pattern of both replacement and diffuse fibrosis. Moreover, they observed a fibrosis gradient from the subendocardium to the mid-myocardium, perhaps suggesting supply-demand ischemia as a contributing factor.
The degree of myocardial remodeling and fibrosis is closely related to hemodynamic markers of myocardial performance, such as end-diastolic pressure and ejection fraction (4). Moreover, multiple histological studies have now demonstrated an association between myocardial fibrosis at the time of AVR and both impaired recovery of left ventricular systolic function and poor long-term outcomes following valve replacement 17, 27, 28, 29. Although it is certainly plausible that myocardial fibrosis might directly contribute to such outcomes, a causal relationship is yet to be demonstrated.
Imaging Modalities for the Assessment of Myocardial Fibrosis
Although myocardial biopsy and histological analysis are still considered the gold standard assessments of myocardial fibrosis, they have several important limitations precluding their routine clinical application. Myocardial biopsy is an invasive procedure that carries an attendant risk of complications (30). Additionally, as only small areas of the myocardium can be sampled, biopsy is prone to sampling error. By contrast, modern imaging techniques, in particular those provided by cardiovascular magnetic resonance (CMR), allow comprehensive, noninvasive assessments of fibrosis across the entire myocardium as well as quantification of its functional consequences (Table 1). These approaches have been used to assess myocardial fibrosis in a range of cardiovascular conditions including aortic stenosis and are described in the following text.
Table 1.
Performance of Different Imaging Modalities in Aortic Stenosis
| Severity | Ventricular Performance | Diffuse Fibrosis | Replacement Fibrosis | Long-Term Prognosis | |
|---|---|---|---|---|---|
| TTE | +++ | +++ | - | - | +++ |
| CT | ++ | ++ | + | + | + |
| CMR | + | +++ | |||
| Native T1 | ++ | + | + | ||
| ECV%/iECV | ++ | + | + | ||
| LGE | - | +++ | +++ | ||
| FT | - | +++ | - | - | + |
CMR = cardiac magnetic resonance; CT = computed tomography; ECV% = extracellular volume fraction; FT = feature tracking; iECV = indexed extracellular volume; LGE = late gadolinium enhancement; TTE = transthoracic echocardiogram.
Cardiac magnetic resonance
CMR provides unparalleled soft tissue characterization and can be used to identify and measure both diffuse and replacement forms of fibrosis in a single scan without the use of ionizing radiation. When utilized together, the CMR techniques described in the following text offer the best available method of capturing the full spectrum of fibrotic changes within the left ventricular myocardium (26).
Late gadolinium enhancement
Gadolinium-based contrast agents (GBCAs) partition into areas of extracellular expansion (myocardial edema, necrosis, infiltration, or fibrosis). Interpretation of delayed imaging using GBCAs requires clear differences in signal intensity between healthy and diseased myocardium in a relatively discrete distribution. Consequently, late gadolinium enhancement (LGE) is an excellent marker of focal replacement fibrosis, but is insensitive for the detection of more diffuse interstitial fibrosis.
LGE is now well established and widely used as a method for detecting replacement myocardial fibrosis in a broad range of cardiovascular conditions such as ischemic cardiomyopathy, nonischemic dilated cardiomyopathy, cardiac sarcoidosis, cardiac amyloidosis, myocarditis, and hypertrophic cardiomyopathy 31, 32, 33, 34, 35, 36, 37, 38. In each condition, replacement fibrosis detected by LGE serves as an independent and powerful predictor of mortality and adverse cardiovascular events. LGE is also the most studied and best validated imaging method for detecting myocardial fibrosis in aortic stenosis. Multiple independent studies have described a noninfarct (or mid-wall) pattern of LGE in patients with aortic stenosis that is distinct from the pattern of scarring seen in other pathologies such as myocardial infarction (Figure 1). On histology, noninfarct LGE co-localizes with microscars and replacement fibrosis, whereas clinical studies have validated it against other markers of left ventricular decompensation and demonstrated a close association with advanced left ventricular hypertrophy, increased myocardial injury, electrocardiographic changes, impaired diastolic and systolic function, and reduced exercise capacity 25, 39, 40, 41. Once noninfarct LGE becomes established, it progresses rapidly. Although the process is arrested by aortic valve intervention, replacement fibrosis appears irreversible once established. Thus, the burden of replacement fibrosis a patient accumulates while waiting for valve intervention persists with them until death (42). The clinical implications are important, as noninfarct LGE is associated with a poor long-term prognosis. Indeed, 5 studies and a recent meta-analysis (43) have confirmed noninfarct LGE to be an independent predictor of mortality, of incremental value to valve assessments, comorbidity, and left ventricular ejection fraction 28, 41, 44, 45, 46 (Table 2).
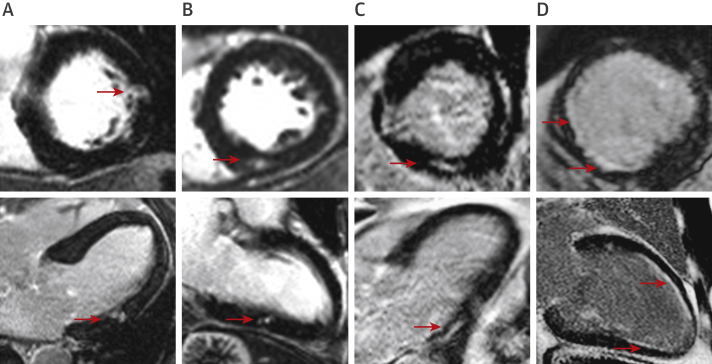
Figure 1.
Late Gadolinium Enhancement Patterns in Aortic Stenosis
Each panel shows short-axis (top) and corresponding long-axis (bottom) late gadolinium images from cardiac magnetic resonance scans. (A to C) Focal noninfarct late gadolinium enhancement typical of the replacement fibrosis seen in aortic stenosis. (D) Subendocardial late gadolinium enhancement in coronary artery territories, consistent with scar due to infarction rather than focal noninfarct fibrosis. Areas of infarction such as these should be excluded when calculating extracellular volume fraction. Red arrows indicate areas of late gadolinium enhancement.
Table 2.
CMR Studies Investigating Myocardial Fibrosis in Aortic Stenosis
| Study (Ref. #) | Year | n | Population | CMR | Biopsy | Findings |
|---|---|---|---|---|---|---|
| Native T1Studies | ||||||
| Bull et al. (55) | 2013 | 109 | Severe AS undergoing SAVR Asymptomatic moderate or severe AS |
1.5-T Native T1 shMOLLI |
19 | Native T1 correlated with CVF (r = 0.65; p = 0.002) and increased with disease severity. |
| Lee et al. (56) | 2015 | 80 | Asymptomatic moderate or severe AS | 3-T Native T1 MOLLI |
20 | Native T1 correlated with histology (r = 0.777; p < 0.001) and TTE measures of diastolic dysfunction, and was increased compared with control patients, with overlap. |
| ECV Studies | ||||||
| Flett et al. (62) | 2010 | 18 | Severe AS undergoing SAVR | 1.5-T ECV% EQ-CMR FLASH-IR |
18 | ECV% correlated with CVF (r2 = 0.86; p < 0.001). |
| Fontana et al. (77) | 2012 | 18 | Severe AS undergoing SAVR | 1.5-T ECV% EQ-CMR shMOLLI FLASH-IR |
18 | ECV% correlated with CVF (r2 = 0.685). ShMOLLI was superior to FLASH-IR. |
| White et al. (66) | 2013 | 18 | Severe AS undergoing SAVR | 1.5-T ECV% EQ-CMR DynEQ-CMR shMOLLI |
18 | ECV% by both methods correlated with CVF (r2 = 0.69; p < 0.01 and r2 = 0.71; p < 0.01). |
| Flett et al. (78) | 2012 | 63 | Severe AS undergoing SAVR | 1.5-T ECV% EQ-CMR FLASH-IR |
— | ECV% was increased compared with control subjects, with overlap. At 6 months, LVH had regressed but diffuse fibrosis was unchanged. |
| LGE Studies | ||||||
| Weidemann et al. (27) | 2009 | 46 | Severe AS undergoing AVR | LGE | 46 | LGE appeared to be concordant with histology (88% with severe fibrosis had ≥2 positive segments; 89% with no fibrosis had no positive segments) and did not regress at 9 months post-AVR. |
| Azevedo et al. (28) | 2010 | 28 | Severe AS undergoing AVR | 1.5-T LGE |
28 | LGE was present in 61%. LGE correlated with histology (r = 0.67; p < 0.001). LGE was an independent predictor of all-cause mortality (HR: 1.26; 95% CI: 1.03–1.54; p = 0.02). |
| Debl et al. (79) | 2006 | 22 | Symptomatic AS | 1.5-T LGE |
— | LGE was present in 27%. LGE correlated with more severe AS and LVH. |
| Rudolph et al. (80) | 2009 | 21 | Any AS | 1.5-T LGE |
— | LGE was present in 62%. LGE correlated with increased LV mass and end-diastolic volume index. |
| Dweck et al. (44) | 2011 | 143 | Moderate or severe AS | 1.5-T LGE |
— | LGE present in 66%. Midwall LGE present in 38%. Midwall LGE was an independent predictor of all-cause mortality (HR: 5.35; 95% CI: 1.16–24.56; p = 0.03). |
| Baron-Rochette et al. (45) | 2014 | 154 | Severe AS undergoing AVR | 1.5-T LGE |
— | LGE present in 29%. LGE was an independent predictor of all-cause mortality (HR: 2.8; 95% CI: 1.1 to 6.9; p = 0.025). |
| Rajesh et al. (81) | 2017 | 109 | Severe AS | 1.5-T LGE |
— | LGE present in 43%. Midwall LGE present in 31%. LGE predicted heart failure/hospitalization and a fall in LVEF but did not predict mortality. |
| Musa et al. (46) | 2018 | 674 | Severe AS undergoing AVR | 1.5-T, 3-T LGE |
— | LGE present in 51%. Noninfarct LGE present in 33%. Scar associated with all-cause (26.4% vs 12.9%; p < 0.001) and cardiovascular (15.0% vs 4.8%; p < 0.001) mortality in a dose-dependent fashion (for every 1% increase in scar, HR: 1.11; 95% CI: 1.05–1.17; p < 0.001 for all-cause and HR: 1.08; 95% CI: 1.01–1.17; p < 0.001 for cardiovascular mortality). Infarct and noninfarct scar were both associated with adverse outcomes. |
| de Meester et al. (82) | 2015 | 12 | Severe AS undergoing SAVR | 3-T Native T1 ECV% LGE MOLLI |
12 | LGE was present in 17 of 31 patients (from total cohort). Only ECV% correlated with histology (r = 0.79; p = 0.011). |
| Kockova et al. (57) | 2016 | 31 | Severe AS undergoing SAVR | 1.5-T Native T1 ECV% MOLLI |
31 | Patient with severe MF (>30%) on histology had higher native T1 times and ECV%. Native T1 ≥1,010 ms and ECV ≥0.32 had AUC of 0.82 and 0.85, respectively, for severe MF. |
| Chin et al. (41) | 2017 | 166 | Any AS | 3-T iECV LGE MOLLI |
11 | Midwall LGE was present in 27%. iECV correlated with histology (r = 0.87; p < 0.001) and was increased compared with control subjects. iECV + LGE predicted unadjusted all-cause mortality (36 vs. 8 deaths/1,000; p = 0.009). |
| Treibel et al. (26) | 2018 | 133 | Severe AS undergoing AVR | 1.5-T ECV% LGE MOLLI |
133 | LGE was present in 60%; noninfarct pattern was more common. Complex MF patterns. LGE, but not ECV%, correlated with CVF in all biopsies (r2 = 0.28; p < 0.001) but more in biopsies with endocardium (r2 = 0.501; p < 0.001). Combined LGE + ECV% best predicted LV remodeling and functional capacity. |
| Child et al. (83) | 2018 | 25 | Severe AS | 3-T Native T1 ECV% LGE MOLLI, shMOLLI, SASHA |
12 | Noninfarct LGE was present in 20%. Sequences differed in discrimination between health and disease as well as association with CVF. Native T1 with MOLLI correlated best (r = 0.582; p = 0.027). |
| Chin et al. (59) | 2014 | 20 | Any AS | 3-T Native T1 ECV% MOLLI |
— | ECV displayed excellent scan-rescan reproducibility and was higher in AS than control subjects. Native T1 was not as reproducible and was not significantly higher in AS than control subjects. |
| Chin et al. (40), Shah et al. (39) | 2014 | 122 | Any AS | 3-T ECV% LGE MOLLI |
— | Midwall LGE was present in 28%. ECV% and LGE were associated with elevated TnI and ECG evidence of strain. |
| Dusenberry et al. (84) | 2014 | 35 | Congenital AS | 1.5-T ECV% LGE Look-Locker |
— | LGE was present in 24%. ECV% was increased compared to control patients and correlated with TTE measures of diastolic dysfunction. |
| Treibel et al. (25) | 2018 | 116 | Severe AS undergoing AVR | 1.5-T iECV LGE MOLLI |
— | At 1 yr, cellular and matrix volume regressed. LGE was unchanged. |
| Everett et al. (42) | 2018 | 99 | 61 asymptomatic AS 38 severe AS undergoing AVR | 1.5-T, 3-T iECV LGE |
— | Midwall LGE was present in 26%. LGE progressed from baseline and was most rapid in patients with more severe stenosis. In patients undergoing AVR, iECV reduced by 11% (4%–16%) but there was no change in LGE. |
| Lee et al. (58) | 2018 | 127 | Moderate or severe AS | 3-T Native T1 LGE MOLLI |
— | LGE was present in 32.3%. Native T1 was increased compared with control patients, with overlap. Native T1 and LGE were independent predictors of poor prognosis. |
AS = aortic stenosis; AUC = area under the curve; CI = confidence interval; CMR = cardiac magnetic resonance; CVF = collagen volume fraction; DynEQ-CMR = dynamic equilibrium contract-cardiac magnetic resonance; ECV% = extra-cellular volume fraction; EQ-CMR = equilibrium contrast cardiac magnetic resonance; FLASH-IR = fast low angle single shot inversion recovery; HR = hazard ratio; iECV = indexed extracellular volume; LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; MOLLI = modified Look-Locker inversion recovery; SASHA = saturation recovery single-shot acquisition; SAVR = surgical aortic valve replacement; shMOLLI = shortened modified Look-Locker inversion recovery; TnI = troponin I; TTE = transthoracic echocardiography.
The poor prognosis associated with non-infarct LGE appears to persist long after AVR is performed, in keeping with the irreversible nature of replacement fibrosis. In the largest study to date, the British Society for Cardiovascular Magnetic Resonance Valve Consortium performed comprehensive CMR assessments in over 650 patients with severe aortic stenosis just prior to SAVR or TAVR (46). At a median follow-up of 3.6 years, LGE (present in 50% of patients) was a powerful independent predictor of all-cause (26.4% vs. 12.9%; p < 0.001) and cardiovascular mortality (15.0% vs. 4.8%; p < 0.001) following AVR. Furthermore, this association appeared dose-dependent: with every 1% increase in left ventricular myocardial scar burden, all-cause and cardiovascular mortality increased by 11% and 8%, respectively (hazard ratio [HR]: 1.11; 95% confidence interval [CI]: 1.05 to 1.17; p < 0.001; and HR: 1.08; 95% CI: 1.01 to 1.17; p < 0.001). Similar effects were observed for both infarct and noninfarct LGE. Noninfarct LGE was also demonstrated to be an independent predictor of both all-cause and cardiovascular mortality.
LGE is reliable, well-validated, and easily integrated into the standard workflow, with post-processing and qualitative analysis readily performed in <10 min in most cases. LGE is therefore ready for investigation as a tool for use in routine clinical practice. Indeed, the ongoing EVOLVED (Early Valve Replacement Guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis) trial (NCT03094143) (47) will investigate whether patients in whom noninfarct LGE is identified may benefit from early AVR before further fibrosis develops and left ventricular decompensation progresses (see the Future Directions section).
T1 mapping
Although LGE is now well-established as a marker of replacement fibrosis, this technique is not able to detect the diffuse interstitial fibrosis that also characterizes left ventricular decompensation in aortic stenosis. Moreover, LGE quantification can be challenging in diffuse fibrotic states. Novel CMR T1 mapping approaches have been developed to overcome these issues. These are reviewed in depth elsewhere 48, 49, but in brief, parametric T1 maps are produced where the tissue T1 time is encoded as signal intensity within each voxel on a static 2-dimensional image and converted to color maps to aid visual interpretation (Figure 2). Native T1 values reflect the state of both the intracellular and extracellular environments, while the addition of a GBCA facilitates targeted interrogation of the extracellular space.
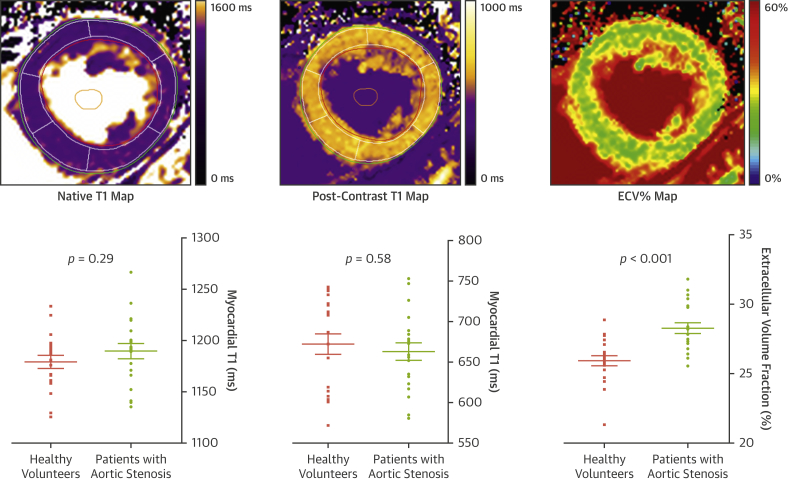
Figure 2.
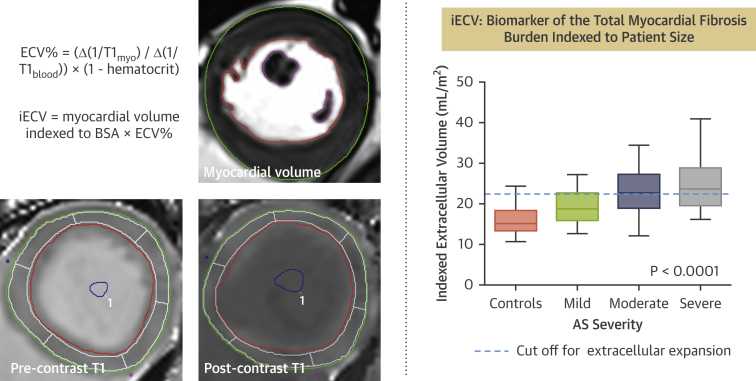
T1 Mapping
Three different cardiac magnetic resonance T1 maps are demonstrated. Native T1 and post-contrast T1 maps are generated by the signal intensity encoded within each voxel, depending on the T1 relaxation time; color coding according to T1 times is applied for visual reference. ECV% maps are generated using the formula ECV% = (Δ[1/T1myo]/Δ[1/T1blood]) × (1 − hematocrit), where Δ(1/T1) is the difference in myocardial or blood T1 pre-contrast and post-contrast. ECV% can be used to assess the proportion of the myocardium comprised by extracellular space. Note that there is significant overlap between health and disease with native and post-contrast T1, in contrast to ECV%. Graphs adapted from Chin et al. (59) by permission of Oxford University Press. ECV% = extracellular volume fraction; iECV = indexed extracellular volume.
Various protocols for T1 mapping have been studied (49). The original Look-Locker technique (50) has been largely superseded by modern variations. The modified Look-Locker imaging sequence (51) is the most studied inversion-recovery technique, whereas variants such as the shortened modified Look-Locker imaging sequence require a shorter breath hold (52). Optimization of protocols has improved accuracy, acquisition time, and ease of use via reduction in heart rate dependence and breath holds. Moreover, post-processing and analysis of T1 mapping data can now be performed with fast and reproducible techniques utilizing standardized protocols. T1 mapping techniques are now readily accessible in many CMR units and will be discussed below.
Native T1
As fibrosis increases, native T1 values increase. Quantitative T1 measurements therefore allow detection of focal or diffuse fibrosis without the use of GBCAs, although the T1 signal also changes with other pathological processes such as edema or myocardial infiltration. Native T1 has been utilized in conditions such as myocardial infarction, myocarditis, dilated cardiomyopathy, cardiac amyloid, and Fabry disease (48), and has demonstrated significant prognostic power beyond that of LGE alone 53, 54. Although less robust, data is also emerging for native T1 in aortic stenosis. Recent studies have demonstrated a correlation between native T1 and both the degree of diffuse fibrosis on histology and the extent of ventricular remodeling on CMR 55, 56, 57 (Table 2). Lee et al. (58) recently presented a single-center cohort of 127 patients with moderate or severe AS in whom native T1 was an independent predictor of heart failure hospitalization or death (2.4% vs. 11.6% vs. 42.9% for low, mid, and high tertiles of native T1, respectively; p < 0.001).
Although native T1 is relatively uniform and reproducible when using the same sequence and scanner on the same patient, values are subject to a variety of factors such as patient age and sex, acquisition sequence, scanner field strength, and post-processing. In aortic stenosis, even within the same scanner and protocol, substantial overlap exists in T1 values across different severities of aortic stenosis and with healthy control subjects (59). Consequently, there are no universal cutoffs for health and disease in aortic stenosis (60). The International T1 Mapping Multicenter Consortium (61) has successfully standardized a multivendor sequence and provided valuable diagnostic and prognostic data in other disease states. However, although native T1 holds major appeal as a marker of diffuse fibrosis that does not require contrast administration and is favored as a technique by various experts in the field, its specific role in aortic stenosis requires further research.
Post-contrast T1 mapping
GBCAs do not cross cell membranes and therefore distribute throughout the extracellular space in the myocardium. Post-contrast T1 mapping techniques therefore allow more specific interrogation of the extracellular space due to gadolinium’s shortening effects on T1 relaxation times. Unfortunately, standardization of post-contrast T1 mapping values is difficult due to variation in gadolinium kinetics between patients and even within the same individual on different days. Standardized normal values are again lacking, and consequently, post-contrast T1 mapping is not in widespread use.
Extracellular volume fraction
The extracellular volume fraction (ECV%) corrects post-contrast myocardial T1 mapping values for blood pool and pre-contrast myocardial T1, thereby accounting for differences in blood concentrations of GBCAs. By incorporating the hematocrit, ECV% calculates the fraction of the myocardium comprised by the extracellular space according to the formula ECV% = (Δ[1/T1myo]/Δ[1/T1blood]) × (1 − hematocrit), where Δ(1/T1) is the difference in myocardial or blood T1 pre- and post-contrast (62). A key feature of myocardial fibrosis is the deposition of excess collagen in the interstitial space and the subsequent expansion of the extracellular space. ECV% has therefore been investigated as a method for detecting diffuse myocardial fibrosis in a range of cardiovascular conditions including myocardial infarction, nonischemic cardiomyopathy, and aortic stenosis 63, 64.
Current scanning techniques assume a dynamic equilibrium between blood and myocardium ∼10 to 15 mins after a bolus injection of contrast 65, 66. A synthetic ECV% has also been described that derives hematocrit from the longitudinal relaxation rate of blood, obviating the need for blood sampling (67), while a more recent noninvasive point-of-care probe to derive hematocrit has demonstrated promising results when compared with both standard and synthetic ECV% (68). ECV% has thus become easier to measure and more clinically applicable. Moreover, ECV% potentially corrects for differences in T1 values on different scanners and sequences, making it appealing as a technique for multicenter research.
A number of clinical studies have validated ECV% against histology in aortic stenosis and have demonstrated the association between ECV% and other markers of LV decompensation, including ECG changes of hypertrophy and strain and elevation in biomarkers such as troponin and N-terminal pro-brain natriuretic peptide 26, 39, 40, 41 (Table 2). ECV% also demonstrates excellent scan-rescan reproducibility (59), while guidelines to standardize post-processing have been developed and recommend that areas of noninfarct LGE are included and areas of infarct LGE excluded from regions of interest in ECV% calculation (69). However, data assessing the prognostic value of ECV% in aortic stenosis are limited, and overlap between disease groups is again observed. In addition, the effect of AVR on ECV% may be somewhat counterintuitive as values can increase after surgery—a weakness of assessing the extracellular component of the myocardium as a fraction of the ventricular mass when both the intracellular and extracellular compartments are undergoing reverse remodeling (25).
Indexed extracellular volume
Whereas ECV% provides a percentage estimate, the indexed extracellular volume (iECV) quantifies the total left ventricular extracellular myocardial volume indexed to body surface area by multiplying ECV% by the indexed left ventricular myocardial volume: iECV = ECV% × indexed left ventricular myocardial volume (Figure 3). Furthermore, cellular volume can be calculated: (1 − ECV%) × left ventricular volume). This can also be indexed to body surface area. In combination with LV mass, ECV% and iECV can together provide an understanding of ventricular remodeling and reverse remodeling with respect to both the cellular and extracellular myocardial compartments. Two studies have utilized iECV or matrix volume as a novel assessment of myocardial fibrosis burden 25, 41, with iECV demonstrating a close association with histological fibrosis assessments. Importantly, iECV appears to provide greater discrimination between disease states than other T1 mapping parameters. Chin et al. (41) demonstrated that a threshold of 22.5 ml/m2 (derived from 37 age- and sex-matched healthy volunteers and defined as 2 SDs above the mean) could be used to differentiate healthy myocardium from diseased myocardium infiltrated by diffuse fibrosis, and in doing so, identify patients with early evidence of left ventricular decompensation and adverse long-term outcome (41).
Figure 3.
iECV calculation
The cardiac magnetic resonance short-axis images provide examples of the pre-contrast and post-contrast contours required to calculate iECV. Systolic and diastolic contours are drawn using the short-axis stack to calculate myocardial volume, which is necessary to derive iECV. Color look-up tables have not been applied to the T1 images. iECV provides a surrogate of the total myocardial fibrosis burden according to the formula demonstrated in the figure. iECV demonstrates good correlation with histological fibrosis burden and severity of aortic stenosis. Graph adapted from Chin et al. (41), Creative Commons Attribution License: https://creativecommons.org/licenses/by/4.0/. BSA = body surface area; other abbreviations as in Figure 2.
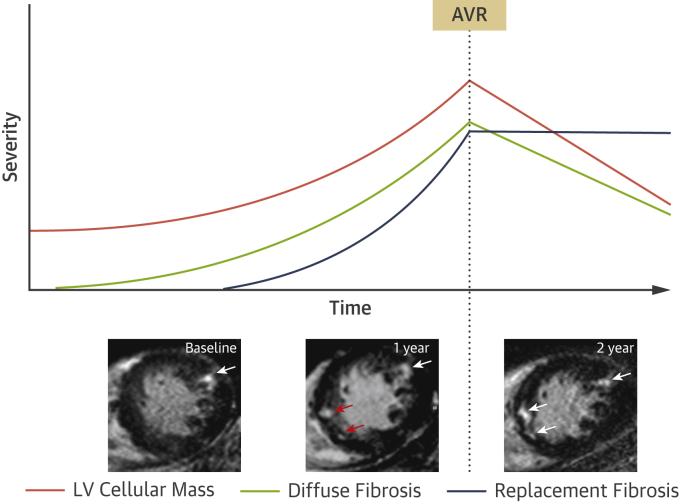
iECV and ECV% have recently been used in combination to study changes in the composition of the intracellular and extracellular compartments before and after AVR. This has provided important insights into left ventricular remodeling and reverse remodeling after relief of loading conditions. Changes in iECV are not accounted for by changes in total left ventricular mass alone. Prior to AVR, iECV (representing total extracellular matrix, or fibrosis, burden) and left ventricular mass appear to increase in a broadly balanced manner so that ECV% remains largely unchanged. Following AVR, left ventricular mass decreases. Cellular and extracellular mass regress, but cellular mass regresses more rapidly, thereby resulting in an apparently paradoxical increase in ECV% as the ratio of matrix to total mass is increased 25, 42. iECV, however, decreases as it represents the extracellular matrix as a total volume, rather than a percentage. The reduction in iECV is therefore in keeping with the potential for reversal of diffuse fibrosis. This effect has been confirmed independently by 2 different groups in separate cohorts and stands in contrast to the irreversible nature of replacement fibrosis as assessed by LGE (Figure 4). iECV requires further exploration and validation but is a promising method to track myocardial fibrosis.
Figure 4.
Schematic for the Development of Myocardial Fibrosis in Aortic Stenosis and Response to AVR
As aortic stenosis progresses, left ventricular (LV) mass gradually increases, followed by the development of diffuse fibrosis. Replacement fibrosis occurs later but accelerates rapidly once established. Following relief of pressure-loading conditions after aortic valve replacement (AVR), LV cellular mass and extracellular matrix both regress at different rates. The burden of replacement fibrosis, however, persists. The insets show short-axis cardiac magnetic resonance late gadolinium enhancement imaging slices of a patient with aortic stenosis. At baseline, there is focal late gadolinium enhancement representing discrete focal replacement fibrosis (white arrow). After 1 year, the burden of this replacement fibrosis has increased with the development of several new discrete deposits (red arrows). The patient subsequently underwent AVR. One year later, despite regression of LV mass, there is no regression of replacement fibrosis (white arrows).
In summary, T1 mapping is an exciting and emerging research field in aortic stenosis research that provides the only method of identifying reversible diffuse myocardial fibrosis. It holds particular potential as a method to track myocardial health over time, with important clinical implications. Standardization of sequences and protocols have resulted in reproducible and powerful prognostic T1 mapping data in a variety of myocardial disease states 41, 53, 54, 58. However, T1 mapping in aortic stenosis is in a relatively early stage of development. Further work is required to establish validated thresholds to aid decision making, paving the way for future multicenter prognostic studies that are ultimately required. Of the T1 mapping parameters currently in use, we believe that ECV% and iECV currently provide the most complete understanding of cellular and extracellular remodeling in aortic stenosis, although native T1 provides important advantages, particularly with regard to ease of calculation and the avoidance of contrast administration.
Other imaging modalities
Alternative imaging techniques to assess myocardial fibrosis in aortic stenosis are limited. Research into computed tomography (CT) assessments of myocardial fibrosis remains exploratory, with only limited data available that has largely focused on measuring ECV on CT scans performed after the administration of iodinated contrast agents (Table 3). These techniques are worthy of further investigation given the widespread use of CT imaging in patients being considered for TAVR and the emerging utility of CT calcium scoring as a marker of stenosis severity. Strain imaging on echocardiography or CMR can be a valuable noninvasive tool to evaluate and quantify myocardial deformation before any identifiable changes in ejection fraction; however, despite an association with imaging markers of myocardial fibrosis 27, 29 and potential prognostic utility 70, 71, this approach is unable to measure myocardial fibrosis directly.
Table 3.
CT to Detect Myocardial Fibrosis
| Study (Ref. #) | Year | n | Population | CT | Biopsy | CMR | Findings |
|---|---|---|---|---|---|---|---|
| Bandula et al. (85) | 2013 | 23 | Severe AS undergoing SAVR | Iohexol equilibrium bolus and infusion protocol | 23 | shMOLLI | ECVCT correlated with ECVCMR (r = 0.73; p < 0.001) and histological fibrosis (r = 0.71; p < 0.001). |
| Hong et al. (86) | 2016 | 20 | Rabbits 4 healthy 16 DCM |
Dual-energy CT Iopamidol bolus |
20 | 3-T MOLLI |
ECVCT correlated with ECVCMR (r = 0.89; p < 0.001) and histological fibrosis (r = 0.925; p < 0.001). |
| Treibel et al. (87) | 2017 | 73 | Validation cohort: 28 severe AS 27 amyloid 18 severe AS underdoing SAVR |
64-detector Iohexol bolus |
18 | — | Good correlation between synthetic and conventional ECVCT (r2 = 0.96; p < 0.001). Good correlation between synthetic and conventional ECVCT and histology (both r2 = 0.50; p < 0.001). ECVCT was higher in amyloidosis. |
| Nacif et al. (88) | 2012 | 24 | 11 healthy 13 HF |
320-detector Iopamidol bolus |
— | 3-T 3(3)5 MOLLI |
Correlation between CMR and CT (r = 0.82; p < 0.001). ECV lower in healthy patients for both CMR and CT (p = 0.03). |
| Nacif et al. (89) | 2013 | 24 | 9 healthy 10 HFrEF 5 HFpEF |
320-detector Iopamidol bolus |
— | — | Mean 3D ECV significantly higher in HFrEF than other groups (p = 0.02). |
| Treibel et al. (90) | 2015 | 47 | 27 severe AS 26 amyloid | 64-detector Iodixanol dynamic equilibrium bolus protocol |
— | 1.5-T shMOLLI | ECVCT at 5 min and 15 min correlated with ECVCMR (r2 = 0.85; r2 = 0.74; p < 0.001). ECVCT was higher in amyloidosis and correlated with markers of severity. |
| Lee et al. (91) | 2016 | 30 | 7 healthy 6 HCM 9 DCM 4 amyloid 4 sarcoid |
Dual-energy CT Iopamidol bolus |
— | 3-T 3(3)5 MOLLI |
Good agreement between ECVCT and ECVCMR on per-subject (Bland-Altman bias 0.06%; 95% CI: 1.19–1.79) and per-segment level. |
CT = computed tomography; DCM = dilated cardiomyopathy; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; other abbreviations as in Table 2.
Future Directions
Myocardial fibrosis is well established as a hallmark pathological feature of left ventricular decompensation in patients with aortic stenosis; yet, it is not routinely assessed in clinical practice. In part, this has reflected the limitations of myocardial biopsy, many of which have now been overcome with advanced noninvasive imaging. The next step is to assess whether these imaging techniques will prove of clinical value in monitoring myocardial health, identifying left ventricular decompensation, and optimizing the timing of AVR.
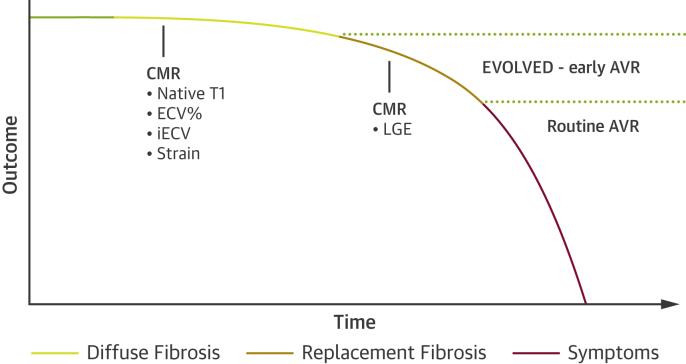
LGE is the best validated of these approaches, is relatively simple to perform and analyze, and is supported by powerful prognostic data. Whether noninfarct LGE can be used to optimize the timing of valve intervention is currently being tested in the EVOLVED (Early Valve Replacement Guided by Biomarkers of LV Decompensation in Asymptomatic Patients With Severe AS) trial (NCT03094143) (47) (Figure 5). This multicenter randomized controlled trial will recruit asymptomatic patients with severe aortic stenosis for CMR imaging. Those patients with noninfarct LGE will then be randomized 1:1 to early valve intervention (SAVR or TAVR) versus the conventional approach of watchful waiting until symptom development or clinical heart failure. To mitigate the costs of CMR, patients will initially be screened with high-sensitivity troponin and an electrocardiogram, both of which are predictors of noninfarct LGE (72); only those patients with an abnormal electrocardiogram or a troponin ≥6 ng/l will proceed to CMR. The primary endpoint is a composite of all-cause mortality and unplanned aortic stenosis–related hospital admissions. This is the first randomized trial to offer targeted early intervention in patients with myocardial fibrosis and left ventricular decompensation, and the results will be of great interest. Similar randomized controlled trials will ultimately be required to establish the clinical utility of other myocardial fibrosis assessments, given that aortic valve intervention is not without risk.
Figure 5.
Proposed Integration of Myocardial Fibrosis Into the Classical Description of the Natural History of Aortic Stenosis
Adaption of the outcome curve originally proposed by Braunwald in 1968 (76). Prior to the onset of symptoms, there is a long latent period in aortic stenosis where subclinical myocardial changes take place, including the development of reversible diffuse fibrosis followed by irreversible replacement fibrosis. These changes may be assessed with the imaging modalities denoted in the figure. Exploratory data suggest that diffuse fibrosis is associated with an adverse long-term outcome in aortic stenosis. The prognostic data related to the noninfarct pattern of late gadolinium enhancement (LGE) as a marker of replacement fibrosis is comparatively robust, establishing LGE as a powerful independent predictor of long-term clinical outcomes. According to current guidelines and routine clinical practice, AVR is performed after the onset of symptoms. Future and ongoing trials, including the EVOLVED trial, are required to determine whether targeted early intervention utilizing cardiac magnetic resonance (CMR) to detect fibrosis will lead to improved clinical outcomes. Abbreviations as in Figures 2 and 4.
CMR assessments of diffuse fibrosis in aortic stenosis require further validation but offer the potential to identify the earlier stages of myocardial disease and track myocardial health with time. T1 mapping is the only available imaging technique that is able to offer an assessment of diffuse fibrosis, and as such, it is crucial that ongoing research is conducted to provide standardization of sequences and protocols across sites and vendors to delineate clear cutoffs for health and disease in aortic stenosis. As T1 mapping research expands, this approach may offer clear advantages over LGE. For example, future investigation of antifibrotic therapies will require biomarkers to monitor myocardial health and treatment effects; T1 mapping will be indispensable in this regard.
Further work to investigate the role of emerging CT techniques is also warranted, particularly as they may be more easily integrated into current clinical care pathways and workflows than CMR. There has also been early investigation of collagen- and elastin-specific CMR contrast agents, which may provide greater contrast to noise ratio compared with current GBCAs, but further advances in this field are awaited 73, 74. Finally, there is considerable interest in developing novel positron-emission tomography tracers to measure myocardial fibrosis activity, in contrast to the structural and functional assessments that have been developed to date. We await further studies to demonstrate this potential. As interest in this field progresses and new techniques emerge, it is of course important to be cognizant of publication bias, which remains an issue in the published medical data (75).
Conclusions
Myocardial fibrosis plays a key role in the pathophysiology of aortic stenosis. Modern imaging techniques now allow assessment of both replacement and diffuse interstitial fibrosis as well as their functional consequences. These techniques hold promise in tracking myocardial health in patients with aortic stenosis, aiding risk stratification and potentially optimizing the timing of aortic valve intervention, with ongoing trials currently testing the clinical efficacy of these approaches.
Footnotes
Dr. Cavalcante has received a research grant from Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Iung B., Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol. 2011;8:162–172. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 3.Osnabrugge R.L., Mylotte D., Head S.J. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Dweck M.R., Boon N.A., Newby D.E. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60:1854–1863. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 5.Rosenhek R., Zilberszac R., Schemper M. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. doi: 10.1161/CIRCULATIONAHA.109.894170. [DOI] [PubMed] [Google Scholar]
- 6.Coffey S., Cox B., Williams M.J. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63:2852–2861. doi: 10.1016/j.jacc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Genereux P., Stone G.W., O'Gara P.T. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2016;67:2263–2288. doi: 10.1016/j.jacc.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 8.Durko A.P., Osnabrugge R.L., Van Mieghem N.M. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39:2635–2642. doi: 10.1093/eurheartj/ehy107. [DOI] [PubMed] [Google Scholar]
- 9.Leon M.B., Smith C.R., Mack M. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 10.Smith C.R., Leon M.B., Mack M.J. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 11.Adams D.H., Popma J.J., Reardon M.J. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 12.Leon M.B., Smith C.R., Mack M.J. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 13.Reardon M.J., Van Mieghem N.M., Popma J.J. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner H., Falk V., Bax J.J. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 16.Krayenbuehl H.P., Hess O.M., Monrad E.S., Schneider J., Mall G., Turina M. Left-ventricular myocardial structure in aortic-valve disease before, intermediate, and late after aortic-valve replacement. Circulation. 1989;79:744–755. doi: 10.1161/01.cir.79.4.744. [DOI] [PubMed] [Google Scholar]
- 17.Hein S., Arnon E., Kostin S. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 18.Yotti R., Bermejo J., Gutierrez-Ibanes E. Systemic vascular load in calcific degenerative aortic valve stenosis: insight from percutaneous valve replacement. J Am Coll Cardiol. 2015;65:423–433. doi: 10.1016/j.jacc.2014.10.067. [DOI] [PubMed] [Google Scholar]
- 19.Lindman B.R., Otto C.M., Douglas P.S. Blood pressure and arterial load after transcatheter aortic valve replacement for aortic stenosis. Circ Cardiovasc Imaging. 2017;10:e006308. doi: 10.1161/CIRCIMAGING.116.006308. [DOI] [PubMed] [Google Scholar]
- 20.Pawade T.A., Newby D.E., Dweck M.R. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. 2015;66:561–577. doi: 10.1016/j.jacc.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 21.Kandalam V., Basu R., Moore L. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation. 2011;124:2094–2105. doi: 10.1161/CIRCULATIONAHA.111.030338. [DOI] [PubMed] [Google Scholar]
- 22.Weber K.T., Brilla C.G. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 23.Heymans S., Schroen B., Vermeersch P. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–1144. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 24.Yang W., Wang B.H., Wang I. Inhibition of apoptosis signal-regulating kinase 1 attenuates myocyte hypertrophy and fibroblast collagen synthesis. Heart Lung Circ. 2017;12:e0187459. doi: 10.1016/j.hlc.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Treibel T.A., Kozor R., Schofield R. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71:860–871. doi: 10.1016/j.jacc.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treibel T.A., Lopez B., Gonzalez A. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J. 2018;39:699–709. doi: 10.1093/eurheartj/ehx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidemann F., Herrmann S., Stork S. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 28.Azevedo C.F., Nigri M., Higuchi M.L. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann S., Stork S., Niemann M. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402–412. doi: 10.1016/j.jacc.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 30.Yilmaz A., Kindermann I., Kindermann M. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–909. doi: 10.1161/CIRCULATIONAHA.109.924167. [DOI] [PubMed] [Google Scholar]
- 31.Weng Z., Yao J., Chan R.H. Prognostic value of LGE-CMR in HCM: a meta-analysis. J Am Coll Cardiol Img. 2016;9:1392–1402. doi: 10.1016/j.jcmg.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Raina S., Lensing S.Y., Nairooz R.S. Prognostic value of late gadolinium enhancement cmr in systemic amyloidosis. J Am Coll Cardiol Img. 2016;9:1267–1277. [Google Scholar]
- 33.Hulten E., Agarwal V., Cahill M. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9:e005001. doi: 10.1161/CIRCIMAGING.116.005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Marco A., Anguera I., Schmitt M. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. J Am Coll Cardiol HF. 2017;5:28–38. doi: 10.1016/j.jchf.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Halliday B.P., Gulati A., Ali A. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation. 2017;135:2106–2115. doi: 10.1161/CIRCULATIONAHA.116.026910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aquaro G.D., Perfetti M., Camastra G. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY Study. J Am Coll Cardiol. 2017;70:1977–1987. doi: 10.1016/j.jacc.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 37.Ganesan A.N., Gunton J., Nucifora G., McGavigan A.D., Selvanayagam J.B. Impact of late gadolinium enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy: a systematic review and meta-analysis. Int J Cardiol. 2018;254:230–237. doi: 10.1016/j.ijcard.2017.10.094. [DOI] [PubMed] [Google Scholar]
- 38.Becker M.A.J., Cornel J.H., van de Ven P.M., van Rossum A.C., Allaart C.P., Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. J Am Coll Cardiol Img. 2018;11:1274–1284. doi: 10.1016/j.jcmg.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Shah A.S., Chin C.W., Vassiliou V. Left ventricular hypertrophy with strain and aortic stenosis. Circulation. 2014;130:1607–1616. doi: 10.1161/CIRCULATIONAHA.114.011085. [DOI] [PubMed] [Google Scholar]
- 40.Chin C.W., Shah A.S., McAllister D.A. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chin C.W.L., Everett R.J., Kwiecinski J. Myocardial fibrosis and cardiac decompensation in aortic stenosis. J Am Coll Cardiol Img. 2017;10:1320–1333. doi: 10.1016/j.jcmg.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everett R.J., Tastet L., Clavel M.A. Progression of hypertrophy and myocardial fibrosis in aortic stenosis: a multicenter cardiac magnetic resonance study. Circ Cardiovasc Imaging. 2018;11:e007451. doi: 10.1161/CIRCIMAGING.117.007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H., Zeng J., Liu D., Yang Q. Prognostic value of late gadolinium enhancement on CMR in patients with severe aortic valve disease: a systematic review and meta-analysis. Clin Radiol. 2018;73:983. doi: 10.1016/j.crad.2018.07.095. e7–14. [DOI] [PubMed] [Google Scholar]
- 44.Dweck M.R., Joshi S., Murigu T. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 45.Barone-Rochette G., Pierard S., De Meester de Ravenstein C. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014;64:144–154. doi: 10.1016/j.jacc.2014.02.612. [DOI] [PubMed] [Google Scholar]
- 46.Musa T.A., Treibel T.A., Vassiliou V.S. Myocardial scar and mortality in severe aortic stenosis. Circulation. 2018;138:1935–1947. doi: 10.1161/CIRCULATIONAHA.117.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dweck M. Early Valve Replacement Guided by Biomarkers of LV Decompensation in Asymptomatic Patients With Severe AS (EVoLVeD) https://clinicaltrials.gov/ct2/show/NCT03094143 Available at: [DOI] [PubMed]
- 48.Puntmann V.O., Peker E., Chandrashekhar Y., Nagel E. T1 mapping in characterizing myocardial disease: a comprehensive review. Circ Res. 2016;119:277–299. doi: 10.1161/CIRCRESAHA.116.307974. [DOI] [PubMed] [Google Scholar]
- 49.Taylor A.J., Salerno M., Dharmakumar R., Jerosch-Herold M. T1 mapping: basic techniques and clinical applications. J Am Coll Cardiol Img. 2016;9:67–81. doi: 10.1016/j.jcmg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Look D.C., Locker D.R. Time saving in measurement of NMR and EPR relaxation times. Review of Scientific Instruments. 1970;41:250–251. [Google Scholar]
- 51.Messroghli D.R., Radjenovic A., Kozerke S., Higgins D.M., Sivananthan M.U., Ridgway J.P. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 52.Piechnik S.K., Ferreira V.M., Dall'Armellina E. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010;12:69. doi: 10.1186/1532-429X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puntmann V.O., Carr-White G., Jabbour A. T1-mapping and outcome in nonischemic cardiomyopathy: all-cause mortality and heart failure. J Am Coll Cardiol Img. 2016;9:40–50. doi: 10.1016/j.jcmg.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Puntmann V.O., Carr-White G., Jabbour A. Native T1 and ECV of noninfarcted myocardium and outcome in patients with coronary artery disease. J Am Coll Cardiol. 2018;71:766–778. doi: 10.1016/j.jacc.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Bull S., White S.K., Piechnik S.K. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S.P., Lee W., Lee J.M. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology. 2015;274:359–369. doi: 10.1148/radiol.14141120. [DOI] [PubMed] [Google Scholar]
- 57.Kockova R., Kacer P., Pirk J. Native T1 relaxation time and extracellular volume fraction as accurate markers of diffuse myocardial fibrosis in heart valve disease- comparison with targeted left ventricular myocardial biopsy. Circ J. 2016;80:1202–1209. doi: 10.1253/circj.CJ-15-1309. [DOI] [PubMed] [Google Scholar]
- 58.Lee H., Park J.B., Yoon Y.E. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. J Am Coll Cardiol Img. 2018;11:974–983. doi: 10.1016/j.jcmg.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Chin C.W., Semple S., Malley T. Optimization and comparison of myocardial T1 techniques at 3T in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2014;15:556–565. doi: 10.1093/ehjci/jet245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Podlesnikar T., Delgado V., Bax J.J. Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease. Int J Cardiovasc Imaging. 2018;34:97–112. doi: 10.1007/s10554-017-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dabir D., Child N., Kalra A. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:69. doi: 10.1186/s12968-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flett A.S., Hayward M.P., Ashworth M.T. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 63.Ugander M., Oki A.J., Hsu L.Y. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong T.C., Piehler K., Meier C.G. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–1216. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller C.A., Naish J.H., Bishop P. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–383. doi: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 66.White S.K., Sado D.M., Fontana M. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. J Am Coll Cardiol Img. 2013;6:955–962. doi: 10.1016/j.jcmg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Treibel T.A., Fontana M., Maestrini V. Automatic measurement of the myocardial interstitium: synthetic extracellular volume quantification without hematocrit sampling. J Am Coll Cardiol Img. 2016;9:54–63. doi: 10.1016/j.jcmg.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Robison S., Karur G.R., Wald R.M., Thavendiranathan P., Crean A.M., Hanneman K. Noninvasive hematocrit assessment for cardiovascular magnetic resonance extracellular volume quantification using a point-of-care device and synthetic derivation. J Cardiovasc Magn Reson. 2018;20:19. doi: 10.1186/s12968-018-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Messroghli D.R., Moon J.C., Ferreira V.M. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI) J Cardiovasc Magn Reson. 2017;19:75. doi: 10.1186/s12968-017-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagata Y., Takeuchi M., Wu V.C. Prognostic value of LV deformation parameters using 2D and 3D speckle-tracking echocardiography in asymptomatic patients with severe aortic stenosis and preserved LV ejection fraction. J Am Coll Cardiol Img. 2015;8:235–245. doi: 10.1016/j.jcmg.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Hwang J.W., Kim S.M., Park S.J. Assessment of reverse remodeling predicted by myocardial deformation on tissue tracking in patients with severe aortic stenosis: a cardiovascular magnetic resonance imaging study. J Cardiovasc Magn Reson. 2017;19:80. doi: 10.1186/s12968-017-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chin C.W., Messika-Zeitoun D., Shah A.S. A clinical risk score of myocardial fibrosis predicts adverse outcomes in aortic stenosis. Eur Heart J. 2016;37:713–723. doi: 10.1093/eurheartj/ehv525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spuentrup E., Ruhl K.M., Botnar R.M. Molecular magnetic resonance imaging of myocardial perfusion with EP-3600, a collagen-specific contrast agent: initial feasibility study in a swine model. Circulation. 2009;119:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.826388. [DOI] [PubMed] [Google Scholar]
- 74.Wildgruber M., Bielicki I., Aichler M. Assessment of myocardial infarction and postinfarction scar remodeling with an elastin-specific magnetic resonance agent. Circ Cardiovasc Imaging. 2014;7:321–329. doi: 10.1161/CIRCIMAGING.113.001270. [DOI] [PubMed] [Google Scholar]
- 75.Song F., Parekh S., Hooper L. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14 doi: 10.3310/hta14080. iii, ix–xi, 1–193. [DOI] [PubMed] [Google Scholar]
- 76.Ross J., Jr., Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 77.Fontana M., White S.K., Banypersad S.M. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson. 2012;14:88. doi: 10.1186/1532-429X-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flett A.S., Sado D.M., Quarta G. Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2012;13:819–826. doi: 10.1093/ehjci/jes102. [DOI] [PubMed] [Google Scholar]
- 79.Debl K., Djavidani B., Buchner S. Delayed hyperenhancement in magnetic resonance imaging of left ventricular hypertrophy caused by aortic stenosis and hypertrophic cardiomyopathy: visualisation of focal fibrosis. Heart. 2006;92:1447–1451. doi: 10.1136/hrt.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudolph A., Abdel-Aty H., Bohl S. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009;53:284–291. doi: 10.1016/j.jacc.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 81.Rajesh G.N., Thottian J.J., Subramaniam G., Desabandhu V., Sajeev C.G., Krishnan M.N. Prevalence and prognostic significance of left ventricular myocardial late gadolinium enhancement in severe aortic stenosis. Indian Heart Journal. 2017;69:742–750. doi: 10.1016/j.ihj.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Meester de Ravenstein C., Bouzin C., Lazam S. Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson. 2015;17:48. doi: 10.1186/s12968-015-0150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Child N., Suna G., Dabir D. Comparison of MOLLI, shMOLLLI, and SASHA in discrimination between health and disease and relationship with histologically derived collagen volume fraction. Eur Heart J Cardiovasc Imaging. 2018;19:768–776. doi: 10.1093/ehjci/jex309. [DOI] [PubMed] [Google Scholar]
- 84.Dusenbery S.M., Jerosch-Herold M., Rickers C. Myocardial extracellular remodeling is associated with ventricular diastolic dysfunction in children and young adults with congenital aortic stenosis. J Am Coll Cardiol. 2014;63:1778–1785. doi: 10.1016/j.jacc.2013.11.066. [DOI] [PubMed] [Google Scholar]
- 85.Bandula S., White S.K., Flett A.S. Measurement of myocardial extracellular volume fraction by using equilibrium contrast-enhanced CT: validation against histologic findings. Radiology. 2013;269:396–403. doi: 10.1148/radiology.13130130. [DOI] [PubMed] [Google Scholar]
- 86.Hong Y.J., Kim T.K., Hong D. Myocardial characterization using dual-energy CT in doxorubicin-induced DCM: comparison with CMR T1-mapping and histology in a rabbit model. J Am Coll Cardiol Img. 2016;9:836–845. doi: 10.1016/j.jcmg.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 87.Treibel T.A., Fontana M., Steeden J.A. Automatic quantification of the myocardial extracellular volume by cardiac computed tomography: synthetic ECV by CCT. J Cardiovasc Comput Tomogr. 2017;11:221–226. doi: 10.1016/j.jcct.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 88.Nacif M.S., Kawel N., Lee J.J. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. 2012;264:876–883. doi: 10.1148/radiol.12112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nacif M.S., Liu Y., Yao J. 3D left ventricular extracellular volume fraction by low-radiation dose cardiac CT: assessment of interstitial myocardial fibrosis. J Cardiovasc Comput Tomogr. 2013;7:51–57. doi: 10.1016/j.jcct.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Treibel T.A., Bandula S., Fontana M. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. J Cardiovasc Comput Tomogr. 2015;9:585–592. doi: 10.1016/j.jcct.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee H.J., Im D.J., Youn J.C. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR Imaging. Radiology. 2016;280:49–57. doi: 10.1148/radiol.2016151289. [DOI] [PubMed] [Google Scholar]