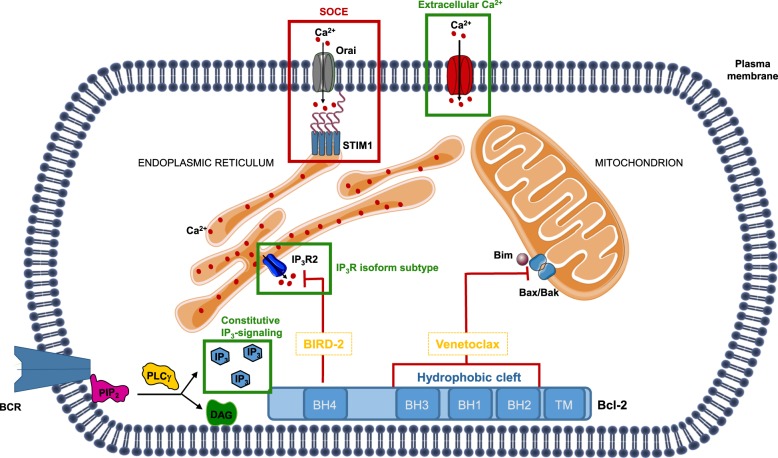
Fig. 1. Antagonizing B-cell lymphoma 2 (Bcl-2) to induce cell death in B-cell cancer cells.
Two functional domains, the hydrophobic cleft and the BH4 domain, are important for Bcl-2’s anti-apoptotic function. The hydrophobic cleft of Bcl-2 prevents apoptosis by scaffolding and neutralizing several pro-apoptotic Bcl-2 family members, including Bax/Bak and BH3-only proteins such as Bim, at the mitochondrial outer membranes. The hydrophobic cleft of Bcl-2 can be targeted by so-called BH3 mimetics, including the recently FDA-approved small molecule venetoclax/ABT-199, provoking cell death in Bcl-2-dependent cancer cells. The BH4 domain suppresses apoptosis by binding and inhibiting the IP3R, intracellular Ca2+-release channels present in the endoplasmic reticulum (ER). A decoy peptide, the Bcl-2 IP3R disruptor-2 (BIRD-2), can target Bcl-2’s BH4 domain, thereby disrupting Bcl-2/IP3R complexes and provoking Ca2+-driven apoptosis in Bcl-2-dependent cancer cells. The IP3R isoform subtype (IP3R2), constitutive IP3 signaling and extracellular Ca2+ are critical factors that contribute to the sensitivity of Bcl-2-dependent cancer cells towards BIRD-2 (indicated in green), while store-operated Ca2+ entry likely may not be involved (indicated in red)