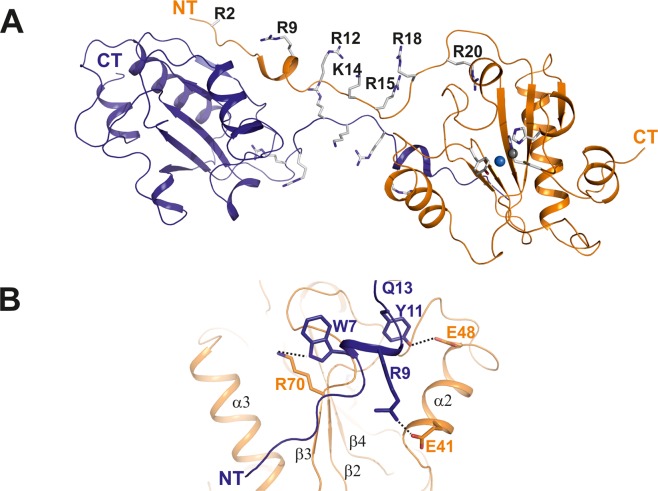
Figure 2.
The dimeric structure of Ts2631 endolysin shows a 3D domain swap via the N-terminus. (A) Asymmetric unit of Ts2631 endolysin showing two protein molecules, which are highlighted in a ribbon representation. The dimer is interconnected by an elongated and largely unstructured N-terminus (residues 1–20), which carries seven positively charged residues (six arginines, one lysine; shown in stick representation), most of which are pointing towards the same direction (residues in stick representation are denoted by type and numbers). The structure represents a 3D domain swap of two interconnected monomers. (B) Molecular interactions between the N-terminus of monomer A (in blue) and the globular domain of monomer B (in orange) are marked by dashed lines. Interacting residues are marked in the same color as their respective domains and numbered according to their position in the sequence.