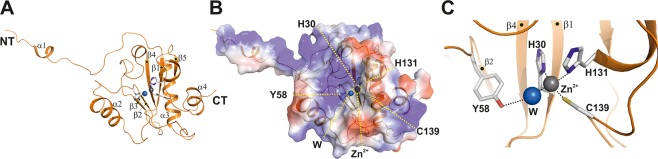
Figure 3.
The crystal structure of Ts2631 endolysin shows a conserved peptidoglycan binding site. (A) Structure of the globular protein presented in a cartoon representation with the extended N-terminal tail comprising a short α-helix, α1 (marked NT and α1). The secondary structure assignment to the mixed α/β structure is α1/β1/β2/α2/β3/β4/α3/β5. The β-sheet is sandwiched between helices α2 and α4, collectively enclosing the active site with the tetragonally coordinated zinc ion (zinc ion represented by a gray sphere; residues are shown in stick representations) in the center of the structure. (B) Surface representation of Ts2631 endolysin demonstrating positively charged patches at the N-terminus and around the active site, which is formed by residues His30, His131, Cys139 and a water molecule (W; blue sphere). (C) Expanded view of the active site in the same perspective as in (A,B), with the active site residues numbered and the zinc ion marked. All structure figures were prepared using PYMOL (www.pymol.org).