Fig. 5.
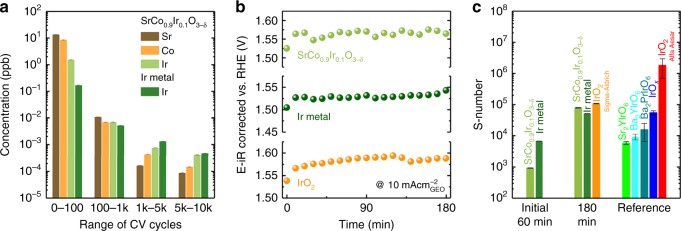
Stability of SrCo0.9Ir0.1O3−δ. a The averaged concentration of dissolved cations per cycle at different stages. The scan rate is 1 V s−1. For the SrCo0.9Ir0.1O3−δ electrode, the potential range is 0.1–1.4 V (vs. RHE). As to the Ir metal electrode, to improve the Ir dissolution, the potential range for the initial 1k cycles and the rest 9k cycles is 0.1–1.6 V (vs. RHE) and 0.1–1.8 V (vs. RHE), respectively. Moreover, a higher overpotential (1.8 V vs. 1.6 V) applied in the rest 9k cycles for Ir metal can facilitate the Ir dissolution41. However, we can see that after 1k cycles, even the upper limit is increased to 1.8 V for Ir metal, there is no significant increase of Ir dissolution within the following 9k cycles. Instead, a lower Ir dissolution is observed, indicating a high instability of Ir at the early stage. b Potential profiles of different electrodes. The chronopotentiometry is performed in 0.1 M HClO4 at 10 mA cm−2, which is normalized to the geometric area of electrodes (GEO). Please note that it does not reflect the intrinsic activity of these materials. c The calculated S-numbers of SrCo0.9Ir0.1O3−δ, Ir metal, and IrO2. The S-numbers of some Ir-based perovskites reported by Geiger et al. are also presented41