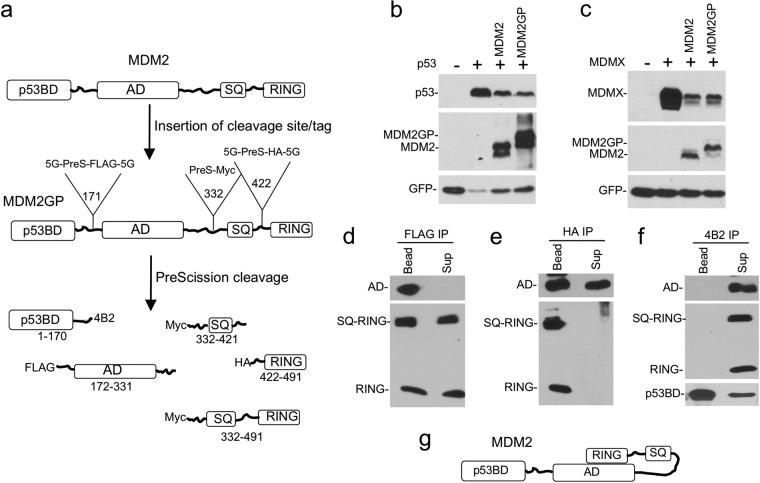
FIG 2.
Construction of a cleavable MDM2 protein. (a) MDM2GP structure. PreScission cleavage site and epitope tags were inserted after residues 171, 332, and 422 of MDM2. (b, c) MDM2GP and MDM2 were cotransfected with p53 (b) or MDMX (c) in H1299 cells. The degradation of p53 and MDMX by MDM2 or MDM2GP was analyzed by Western blotting. (d) MDM2GP was immobilized on beads using anti-FLAG antibody and cleaved with PreScission for 1 h. SQ-RING and RING fragment dissociation from the immobilized AD was detected by HA Western blotting. IP, immunoprecipitation; Sup, supernatant. (e) MDM2GP was immobilized using HA antibody and cleaved with PreScission. AD fragment dissociation from the immobilized RING domain was detected by FLAG Western blotting. (f) MDM2GP was immobilized using anti-4B2 antibody and cleaved with PreScission. AD and RING fragment dissociation from the immobilized p53BD was detected by FLAG and HA Western blotting. (g) Model of intramolecular interaction between MDM2 AD and RING domain. SQ designates the region with multiple ATM phosphorylation sites (residues 386 to 429).