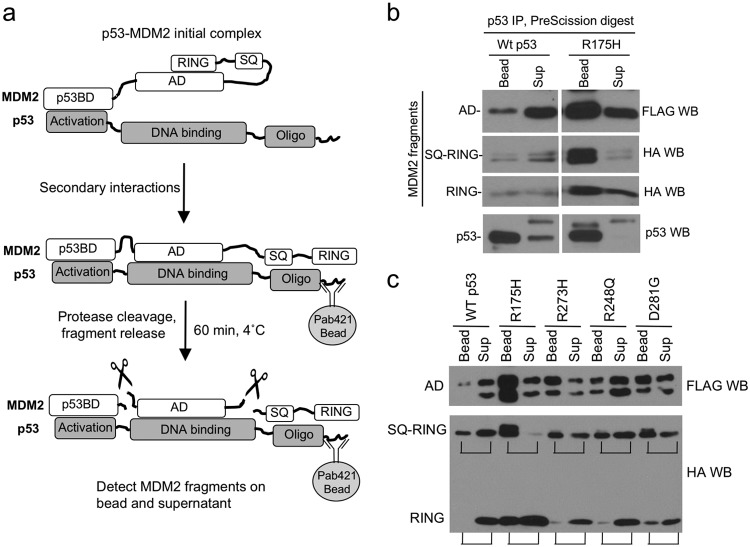
FIG 3.
Increased binding of mutant p53 to MDM2 AD and RING domains. (a) Diagram of proteolytic fragment release (PFR) assay for detecting intermolecular interactions. MDM2GP-p53 complex from transfected H1299 cells was immobilized using anti-p53 antibody Pab421 conjugated to protein A beads. MDM2GP was cleaved by PreScission on the beads, and the release of MDM2 fragments was detected by Western blotting. (b) Comparison of MDM2 domain interactions with wild-type (Wt) and mutant p53. Wild-type p53 or R175H mutant was cotransfected with MDM2GP in H1299. MDM2GP-p53 complex was immobilized by Pab421 beads and cleaved by PreScission. MDM2 fragments that remained bound to the beads or dissociated into the supernatant were analyzed by Western blotting (WB). (c) Comparison of MDM2 domain interactions with wild-type and additional p53 mutants using the fragment release assay.