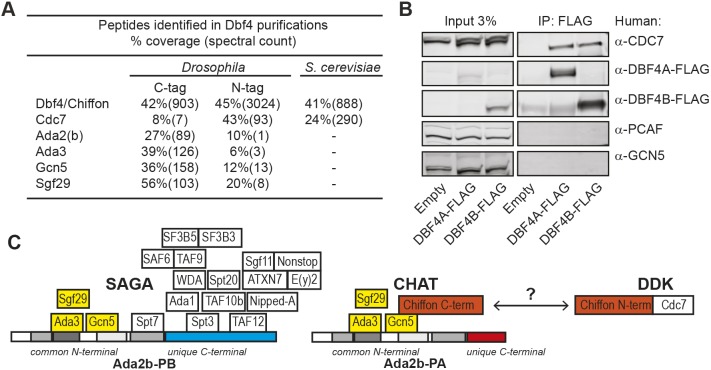
Fig. 3.
Dbf4 does not bind Gcn5 in yeast or humans. (A) Table showing proteins identified in Chiffon and Dbf4 purifications from Drosophila melanogaster (tandem FLAG-HA) or Saccharomyces cerevisiae (TAP-tagged). Sequence coverage (percentage) and number of spectra are shown for each protein. (B) FLAG-tagged human DBF4A or DBF4B were immunoprecipitated from HEK293T cell extracts using anti-FLAG antibodies, and analyzed by western blotting using the indicated antibodies. Control, empty vector. Representative data from three experiments are shown. (C) Schematic showing subunit composition of the SAGA, CHAT and DDK complexes. Interactions between subunits are based on the yeast two-hybrid analysis from Figs S1 and S3, which suggest that Ada2b-PB binds Spt3 and TAF12 via its unique C-terminal domain to nucleate SAGA formation. In contrast, CHAT formation is nucleated by the binding of Chiffon's C-terminal to Gcn5, which precludes association of other SAGA subunits. Chiffon interacts with Cdc7 via its N-terminal domain to form the DDK complex, and the DDK and CHAT complexes appear to be largely separate in vivo.