Figure 4.
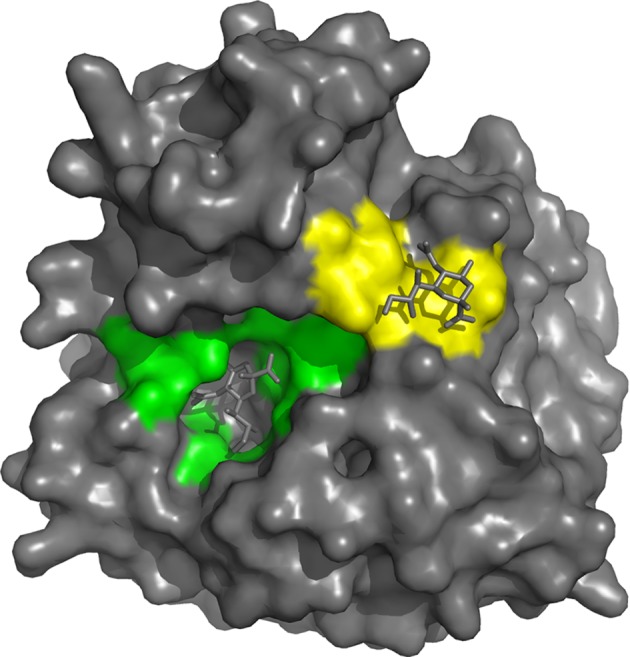
On the basis of interaction with sialic acid, residues Arg118, Asp151, Arg152, Arg224, Glu276, Arg292, Arg371 and Tyr406 are considered as the catalytic sites that mediate cleavage from the underlying sugar residues presented by glycoproteins and are highlighted in green. The second site binds sialic acid by making contacts with Ser367, Ser370, Ser372, Asn400, Trp403 and Lys432 (highlighted in yellow), but bound sialic acid at this site is not released by the NA activity. The structure was generated in Pymol using structural information from Protein Data Bank code 1MWE for the A/tern/Australia/G70C/75 NA/Neu5Ac complexed at 4 °C.
