Figure 4. Structural displacements are distributed throughout both α- and β-tubulin subunits, with enhanced flexibility apparent in α1B/β2B microtubules.
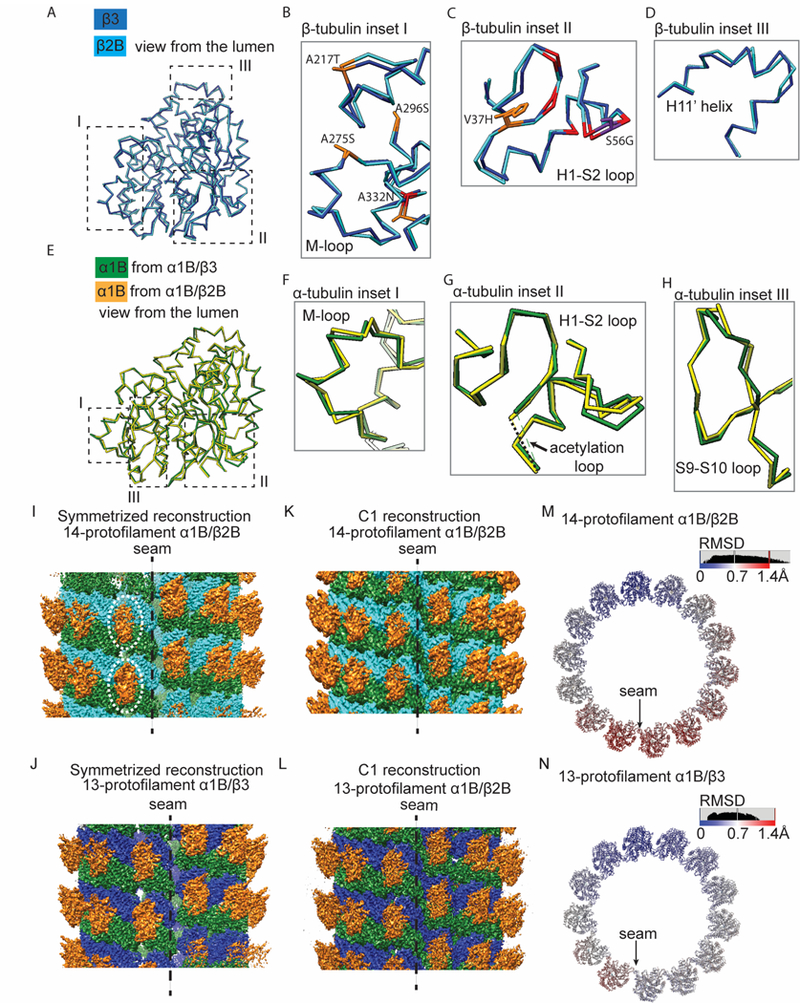
(A) Comparison of the Cα traces of β2B- (cyan) and β3-tubulin (blue). Regions I, II and III are highlighted (dashed boxes). (B-D) Enlarged views of Cα traces of β2B- (cyan) and β3-tubulin (blue) from regions highlighted in (A): around the M-loop (B), the H1-S2 loop (C) and the H11’ helix (D). Colors highlight β3-tubulin hydrophobic residues replaced by polar residues in β2B-tubulin (orange), β3-tubulin polar residues replaced by hydrophobic residues in β2B-tubulin (purple), and other divergent residues (red). (E) Comparison of the Cα traces of α1B-tubulin in 14-α1B/β2B (yellow) and 13-α1B/β3 (green) microtubules. Regions I, II and III are highlighted. (F-H) Enlarged views of Cα traces from regions highlighted in (E): around the M-loop (F), the H1-S2 loop (G) and the S9-S10 loop (H). (I-L) Overviews of the pseudo-helical symmetrized (I and J) and C1 (K and L) cryo-EM reconstructions of kinesin-1 motor domain decorated GMPCPP-14-α1B/β2B (I and K) and GMPCPP-13-α1B/β3 (J and L) microtubules at the seam. The less defined densities of kinesin motors at the seam of the symmetrized GMPCPP-14-α1B/β2B reconstruction are highlighted (dashed circles). α1B-tubulin: green; β2B-tubulin: cyan; β3-tubulin: blue; kinesin-1 motor domain: orange. (M and N) Cα-atom RMSD between superimposed models from the C1 and the pseudo-helical symmetrized reconstructions of 14-α1B/β2B (M) and 13-α1B/β3 (N) microtubules. One helical turn of αβ-tubulin heterodimers is shown. See also Figure S4.
