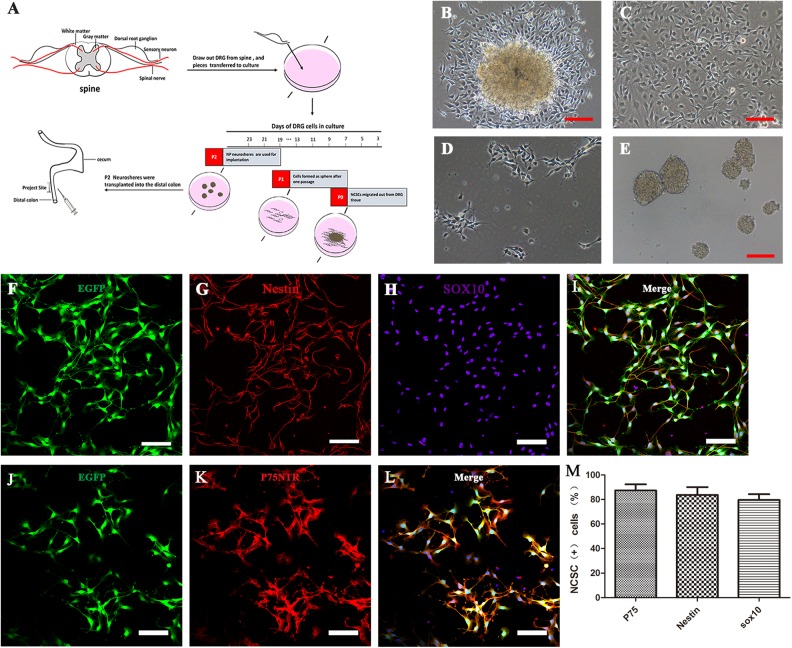
Fig 1.
DRG-derived neural crest stem cells isolation, proliferation, and characterization in vitro. (A) Schematic representation of the workflow of DRG-NCSC derivation, proliferation, and transplantation into the distal intestine of postnatal mice. Pieces of mouse lumbar DRGs were placed on the 24-well plate in the primary medium. Cells migrated out after 1–2 days and were designated as passage 0 cells. Spheres formed after cells were transferred onto low-attachment plates. The spheres about 40 µm in diameter were harvested for transplantation into the distal colon. The cell culture in vitro took roughly 1520 days prior to transplantation. (B–E) Transplantable NCSC cell derivation from DRG explants, proliferation, passage and sphere formation. Phase contrast micrographs taken at different time points in the cell culture. (B) Cells migrated out of the DRG explants and formed a dense layer on the collagen after 1–2 days. (C) Cells that migrated out of the DRG explant proliferated almost to confluence until the next passage. (D) Cells in the primary culture were dissociated into single cells and re-seeded at 1 × 104 cells/cm2 as a monolayer on 6-well plates. (E) Cells were passaged and formed spherical aggregates on low-attachment plates in NPPM. These spheres were used for transplantation into the distal colon of mice. Scale bars, 100 μm. (F–L) DRG-derived NCSCs with EGFP fluorescence (F, J) cultured as primary culture passage were immunostained with the NSC marker Nestin (G) and the NCSC markers Sox10 (H) and P75 (K). Scale bars, 100 μm. (M) Most cells had immunoreactivity to the NCSC markers P75, Sox10 and the NSC marker Nestin as the expression proportion showed in the histogram.