Abstract
Cervical liquid-based cytology plays an important role in the diagnosis of cervical squamous intraepithelial lesion (SIL). However, cytological evaluation alone has a relatively low sensitive. To overcome this problem, HPV DNA testing or HPV DNA combined with cytology has been applied. HPV DNA testing significantly improved the sensitivity, but the specificity is low, especially in cancer and high-grade SIL (HSIL) cases. The aim of this study was to evaluate the diagnostic utility of p16 overexpression in cervical cells of patients with HSIL and cancer. The expression of p16 was detected by immunostaining in liquid-based cells from cervical brushing in 278 patients which including: Cancer (n = 13), HSIL (n = 112), low-grade SIL (LSIL) (n = 45), and Benign (n = 108). The expression levels of p16 were significantly higher in the cancer and HSIL groups when compared with the LSIL and Benign groups (P < 0.01). The accurate diagnostic rates of cancer and HSIL were significantly increased by p16 immunostaining plus cytology than that by cytology alone (P < 0.01). The false negative or false positive of p16 immunostaining occurred with a unicellular pattern. With sensitivity of 96.0% and accuracy of 91.7%, the diagnostic performance of p16 immunostaining was much better than that of cytology alone with sensitivity of 36.0% and accuracy of 70.9% (P < 0.01). p16 immunostaining in cervical brushing cells may not only be used as an ancillary tool to cytological diagnosis of cervical neoplasia but also help to distinguish HSIL from LSIL and the triage of transient infection.
Keywords: p16 immunostaining, low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion, human papellomavirus, liquid-based cytology
Introduction
The cytological concept of cervical high-grade squamous intraepithelial lesion (HSIL) was first proposed by authors of TBS (The Bethesda system for reporting cervical/vaginal cytologic diagnoses) in 19891, which included cervical intraepithelial neoplasia (CIN)2 and CIN3 that determined by histological biopsy. This is based on the fact that the biological behavior and treatment principles of CIN2 and CIN3 are basically the same, and the reproducibility of differentiating them is poor. In 2012, the lower anogenital squamous terminology standardization (LAST), and in 2014 the World Health Organization (WHO) also, recommended using the histological terminology low-grade and high-grade squamous intraepithelial lesion (LSIL and HSIL), respectively, for reporting human papillomaviruses (HPV)-related squamous lesions2,3. This two-tiered system of LSIL and HSIL is superior to the three-tiered system of CIN1, CIN2, and CIN3, which reflects our latest knowledge of HPV pathogenesis: LSIL is associated with transit infection and low-risk progression, while HSIL is associated with persistent infection and high risk of progression to cancer4,5. In current histological pathology practice, traditional CIN2 cases are further classified based on p16 expression: p16-positive cases are classified into HSIL, while negative cases are classified into LSIL. This CIN2/p16-negative LSIL does not require immediate clinical intervention, but only close follow-up. This important role of p16 immunostaining is not widely adopted in cytopathological practice.
P16, a cyclin-dependent kinase inhibitor, plays an important role in cell cycle regulation by decelerating cells progression from G1 phase to S phase. It is usually expressed at low concentration in healthy cells, but is overexpressed in the cervical cell of both HSIL and cancer6,7. Normally, p16 inhibits CDK4/6 that phosphorylates retinoblastoma protein (pRB). Once phosphorylated, pRB disassociates from the transcription factor E2F (E2F), and then E2F enters the nucleus and promotes the transcription of target genes that are essential for transition from G1 to S phase. Thus, p16 acts as a tumor suppressor by binding to CDK4/68. In the cells with HPV infections, viral oncoprotein E7 integrates with host genome and binds with pRB. Once pRB function is inactivated, E2F is released from the sequestration of p16, enters the nucleus, and accelerates cells progression from G1 phase to S phase9. Consequently, functional inactivation of pRB leads to reflex upregulation of p16 by a negative transcriptional feedback mechanism10,11. There is a tight relationship between the overexpression of p16 and the activation of E7 protein. Furthermore, the overexpression of p16 is used as a good indicator for high-risk HPV (hrHPV) persistent infections in vivo.
More and more publications reported that p16 immunostaining plays an important role in the distinction of HSIL from LSIL, as well as in differentiating persistent infections from transient infections of hrHPV12–14. Thus, the diagnosis of cervical HSIL has been developed from simple morphological diagnosis to morphology combined with molecular detection, and the combination has significantly increased the accuracy of diagnosis13,15,16. Currently, p16 immunostaining is limited to cervical biopsies or excision samples; both are invasive procedures. Cervical brushing is a minimally invasive procedure. Once the cytology result is negative, which happens in the majority of patients, no subsequent biopsies are needed. The aim of this study was to evaluate the impact of combined cytolomorphology and p16 immunostaining in liquid-based samples for cervical cancer and HSIL diagnosis.
Materials and Methods
Patient Recruitment and Sample Collection
The study was conducted according to the guidelines of the institutional review boards at the First Hospital of China Medical University, and we have obtained internal review board approval and/or patients’ informed consent for this study. A total 125 patients with Cancer and HSIL and an additional 153 randomly selected patients with LSIL and Benign were recruited for this study. The cervical brushing cell samples were collected by the laboratory of cytopathology in the First Hospital of China Medical University during the period March 1, 2017–March 23, 2017. The ages ranged from 24 to 87 years old with an average age of 45.46 years. All patients in this study had both cytological and histological (biopsy or postoperative biopsy) diagnoses; the comparison of the two diagnostic results is shown in Table 1. The study subjects with LSIL and Benign by histological diagnoses were followed up for 1 year.
Table 1.
Comparison of Histological Diagnosis with Cytological Diagnosis.
| Cytological diagnosis | |||||||
|---|---|---|---|---|---|---|---|
| Histological Diagnosis | n | Cancer | HSIL | ASC-H | LSIL | ASC-US | NILM |
| Cancer | 13 | 2 | 8 | 3 | |||
| HSIL | 112 | 35 | 41 | 9 | 11 | 16 | |
| LSIL | 45 | 2 | 22 | 12 | 9 | ||
| Benign | 108 | 1 | 8 | 2 | 97 | ||
| Total | 278 | 2 | 44 | 54 | 31 | 25 | 122 |
Cancer, invasive carcinoma; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy; Benign, no intraepithelial lesion.
Cytology Preparation, Staining, and Screening
Liquid-based cytology technology was used for cytological preparation. Two slides were automatically prepared for all 278 cases (BD Tripath, Burlington, NC, USA); one was used for the Papanicolaou method stained using SurePath. The other slide was for p16 immunocytochemical staining. The cytological diagnosis was assessed to two independent cytologists. The results were interpreted according to the 2015 Bethesda System.
Immunocytochemical Staining
Slides were stained in an automated immunostainer (Automated immunocytochemical staining system; Jiang Yuan Medical, Guangzhou, China), according to the manufacturer’s protocol. p16 (Anti-CDKN2A/p16INK4a, act# ab189302) antibody was purchased from Abcam (Cambridge, MA, USA) and used at 1:500. Two independent cytopathologists blindly assessed the immunostaining results. The key for the evaluation was: nuclear with/out cytoplasmic staining was considered as positive cells. Negative results were defined as confined cytoplasmic staining, and weak staining that was almost identical to background. Staining intensity was not taken into account in determining a p16-positive result. The p16-positive slides were carefully evaluated according to the four criteria of nuclear score proposed by Wentzensen et al17. These four criteria are: Increased nuclear/cytoplasmic ratio, Chromatin granule, Irregular nuclear shape, and Anisonucleosis. p16-positive cells without any further sign of nuclear alterations were given a score of 1. Cells with mild nuclear abnormalities that displayed only one of the features mentioned above were given a score of 2. Cells with an increased nuclear/cytoplasmic ratio (> 50%) and with one additional positive criterion were given a score of 3. All cells with an increased nuclear/cytoplasmic ratio and more than one additional positive criterion were given a score of 4. A score of 0 was rated for cases without any p16-positive cells. Any one nucleus with score >2 was regarded as positive. According to the distribution patterns of p16-positive cells, each case was further categorized into three groups: Flake, Patchy, and Unicellular. The Flake group was defined by cells’ distribution as flake clusters with most of cells positive; the Patchy group was defined by cells distributed as alternating clusters by either positively or negatively stained cells with a few cells positive; the Unicellular group was defined by single-cell positive cells.
Colposcopy and Histological Diagnoses
All patients positive for HPV16 or 18 were examined by colposcopy and underwent cervical biopsy; biopsy samples were obtained within 4 weeks after the initial HPV DNA tests. Histological diagnosis was made by two experienced pathologists. The histological biopsy results were categorized into four general groups: Benign (including no pathologic alteration and benign or reactive changes), LSIL (including CIN1 and CIN2 of p16-), HSIL (including CIN2 of p16+, CIN3, and squamous cell carcinoma in situ, and/or involving glands), and Cancer (invasive carcinoma). In patients who had more than one tissue sample, the highest grade diagnosis was recorded.
Statistical Analysis
The SPSS 16.0 statistical software package (SPSS, Inc. Chicago, IL, USA) was used for all data analyses. The chi-square test or Fisher’s exact test was used to compare expression of p16 between each two different groups. Diagnostic utility for p16 expression results were calculated and compared with those for cytological results. For result comparison between p16 imunostaining and cytology, we used McNemar’s test. The diagnostic performance of p16 imunostaining and cytology were assessed by computing sensitivity, specificity, and accuracy. The level of statistical significance was set at P < 0.05.
Results
The Results of p16 Immunostaining and Cytological Diagnosis
Table 1 showed the comparison of cytological and histological diagnostic results. The study patients with LSIL and Benign by histological diagnoses were followed up for 1 year. The results of p16 immunostaining and cytological diagnosis in cervical cells of patients with carcinoma, dysplasia, and no intraepithelial lesion are presented in Table 2. The positive immunostaining expression levels of p16 were significantly higher in cancer and HSIL groups than that in LSIL and Benign groups (P < 0.01).
Table 2.
Results of p16 Detection by Immunocytochemistry Compared with Cytological Assessment According to Histological Diagnosis.
| Histology | n | Cytology | p16 | ||
|---|---|---|---|---|---|
| Diagnosis | n | + | — | ||
| Cancer | 13 | Cancer | 2 | 2 | 0 |
| HSIL | 8 | 8 | 0 | ||
| ASC-H | 3 | 3 | 0 | ||
| HSIL | 112 | HSIL | 35 | 35 | 0 |
| ASC-H | 41 | 41 | 0 | ||
| LSIL | 9 | 9 | 0 | ||
| ASC-US | 11 | 10 | 1 | ||
| NILM | 16 | 12 | 4 | ||
| LSIL | 45 | ASC-H | 2 | 1 | 1 |
| LSIL | 22 | 6 | 16 | ||
| ASC-US | 12 | 1 | 11 | ||
| NILM | 9 | 5 | 4 | ||
| Benign | 108 | HSIL | 1 | 0 | 1 |
| ASC-H | 8 | 1 | 7 | ||
| ASC-US | 2 | 0 | 2 | ||
| NILM | 97 | 4 | 93 | ||
| Total | 278 | 278 | 138 | 140 | |
Carcinoma, invasive carcinoma; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy; Benign, no intraepithelial lesion.
Detailed Comparison of p16 Immunostaining and Cytological Diagnosis
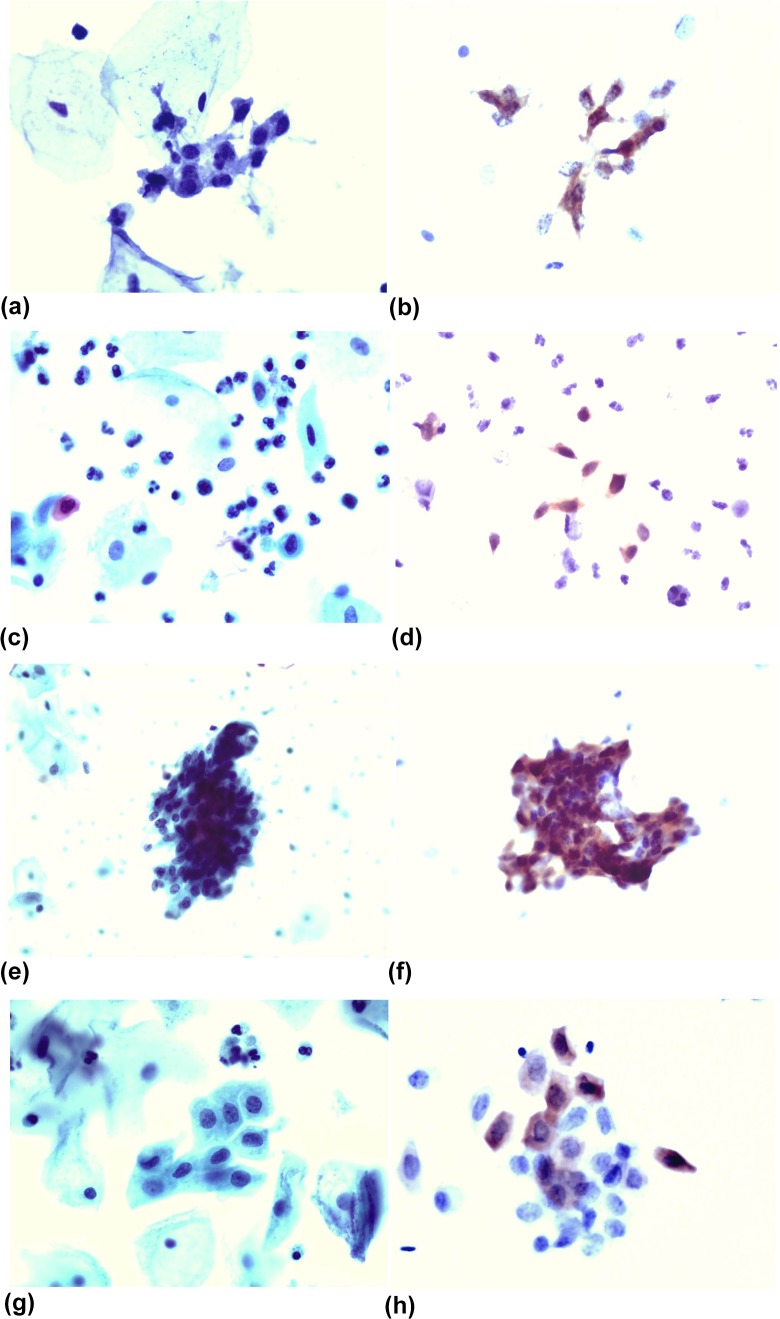
Table 2 shows a more detailed comparison between the cytological screening results and the p16 immunostaining results. In the 13 cases of Cancer, three cases were cytologically diagnosed as ASC-H, while p16 immunostaining showed positive results. In 112 cases of HSIL, only 35 cases that the cytological diagnosis were consistent with histological results. But by p16 immunostaining, 107 cases were positive (Fig. 1A and B). Thus, the diagnostic rates for cancer and HSIL were significantly increased by p16 immunostaining compared with cytology alone (P < 0.01). In the LSIL and Benign groups, there were a few lesions also positive for p16 immunostaining; however, there was no statistical significance. The pattern of p16-positive cells provided some clue for identifying a false negative. We found that most false negatives for p16 expression occurred with a Unicellular pattern (Fig. 1C and D).
Figure 1.
(a) A slide prepared by the LBP method, showing a cluster of small cell type HSIL cells that are easily overlooked (Papanicolaou stain, ×400). (b) A slide from the same patient immunostained with p16, showing that the HSIL cells are easy to interpret because of the obviously positive nucleus. (c) A slide prepared by the LBP method, showing some scattered HSIL cells that are easily overlooked (Papanicolaou stain, ×400). (d) A slide from the same patient immunostained with the p16, showing that the HSIL cells are easy to interpret because the nucleus is obviously positive. (e) A slide prepared by the LBP method, showing a cluster of HCGs-type HSIL cells that are easily misinterpreted (Papanicolaou stain, ×400). (f) A slide from the same patient immunostained with the p16, showing that the HSIL cells are easy to interpret because the nucleus is obviously positive. (g) A slide prepared by the LBP method, showing a cluster of metaplastic-type HSIL cells that are easily misinterpreted (Papanicolaou stain, ×400). (h) A slide from the same patient immunostained with the p16, showing that the HSIL cells are easy to interpret because the nucleus is obviously positive.
Diagnostic Performance of p16 Immunostaining and Cytological Diagnosis
Table 3 showed the comparison of diagnostic accuracy between p16 immunocytochemistry and cytology (based on histological diagnosis of cervical dysplasia and carcinoma). According to the triage principle of the cervical intraepithelial lesion, HSIL requires intervention, while LSIL usually takes follow-up observation. We used Cancer and HSIL as positive cases, and LSIL and Benign as negative cases. The diagnostic efficiency of cytological screening and p16 detection was then calculated. With sensitivity 96.0% and accuracy 91.7%, the diagnostic performance of p16 immunostaining was much better than that of cytology alone with sensitivity 36.0% and accuracy 70.9% (P < 0.01).
Table 3.
Accuracy of p16 Detection by Immunocytochemistry Compared with Cytology for the Histological Diagnosis of Cervical Dysplasia and Carcinoma.
| Methods | P16 | Cytology | P16 plus Cytology | |
|---|---|---|---|---|
| Sensitivity | % (±95%CI) | 96.0 (±3.44)* | 36.0 (±8.41) | 96.0 (±3.44)* |
| Specificity | % (±95%CI) | 88.2 (±5.11)* | 99.3 (±1.28) | 100 (±0.00) |
| Accuracy | % (±95%CI) | 91.7 (±3.24)* | 70.9 (±5.34) | 98.2 (±1.56)* |
CI, confidence intervals; *P < 0.01 as compared with cytology.
Discussion
Cervical cytology is an important method for cervical cancer screening, and is also the main triage tool for cervical intraepithelial lesion18. However, in the real world, the screening is not only for cervical cancer, but also for cervical precancerous lesions. The identification of cervical cancer means that the lesion is already in the stage of invasion, the survival rates of patients are significantly reduced, and the goal of early diagnosis and early treatment is missed. Therefore, the most important aim of cervical cytology is to identify the key target HSIL for early treatment.
One major advantage of cytology is that it is minimally invasive when compared with the invasive procedures of surgical biopsy. However, the obvious disadvantages of cytology screening are low sensitivity19 and its inability to further differentiate whether CIN2 lesions belong to HSIL or belong to LSIL20. In our cohort, many HSIL cases but only few LSIL cases were misinterpreted by cytology. The misinterpretation of HSIL is partly because of the small size of HSIL cells, and the 3D crowded hyperchromatic clusters make evaluation of individual cells difficult21. HSIL cells are easily missed (false negative results) when they coexist with LSIL cells, metaplastic cells, repair cells, and atrophic cells; Solomon et al. reported that an estimated 15% of HSIL usually were hidden in the LSIL group21. In our study, 77 out of the 112 HSIL cases were misinterpreted as ASC-H (41), LSIL (9), ASC-US (11), and NILM (16) (Table 1) by cytological evaluation alone; but 72 out of 77 HSIL cases were correct when p16 immunostaining was incorporated into the cytological evaluation. Many countries apply HPV DNA or HPV DNA combined with cytology to improve the sensitivity of cervical cancer screening. However, the HPV DNA test is only a qualitative test. It is not able to distinguish the severity of lesions (HSIL vs. LSIL), nor differentiate transit infection from persistent infection. Consequently, the HPV DNA test is not helpful for making the decision for intervention or close follow-up. Because the cytomorphology of HSIL and atrophic cells is sometimes quite similar, cytological diagnosis based on morphology alone may be a diagnostic challenge, especially when the atrophic cells present as hyperchromatic crowded groups (HCGs) of cells and are easily mistaken for HSIL. In our study, 9 out of the 108 Benign cases were misinterpreted as HSIL(1) and ASC-H (8) (Table 1) by cytological evaluation alone; but 8 out of 9 Benign cases were correctly identified when p16 immunostaining was incorporated into the cytological evaluation. Therefore, a reliable detection method is urgently needed to assist the cytological screening of HSIL cells.
Both LAST and WHO recommend that overexpression of p16 should be used as an important marker for HSIL or worse lesions. For the category of CIN2 cases, HSIL must be differentiated from LSIL by p16 immunostaining, not by morphology alone2,3. In the present study, the sensitivity of screening HSIL or worse lesion by p16 was significantly increased compared with cytology alone. Our results indicated that p16 is an ideal marker for screening HSIL or worse lesion.
In some cases of HSIL that are difficult to diagnose, p16 plays an important role for decision making. For example, in HCGs type, most of the cells are crowded and overlapped, so the cell’s nuclear membrane and the nuclear outline cannot be clearly displayed. Even using the method of regulating the microhelix of microscope or by observation of the cells at the HCG’s margins following the TBS guide, the results are still not satisfactory22. In our study, 37 cases were cytologically diagnosed as HCGs type out of 41 cases of ASC-H (Fig. 1E). The small or single scattered HSIL cells are very easily overlooked, especially under low magnification or when cytologists are visually fatigued. In this study, 16 HSIL cases were misdiagnosed as NILM by cytological observation only, and all 16 cases were small or single scattered HSIL cells in the distribution type. In addition, when HSIL cells, LSIL cells, metaplastic cells, repair cells, and atrophic cells coexist, HSIL cells (Fig. 1G, metaplastic type) were very easily missed because of the interference from other cells. In the present study, 17 HSIL cases were diagnosed coexisting with other cells in LSIL or ASC-US (total 20 cases). However, p16 detection did not omit any lesion cells (Fig. 1F) and there was no interference by any factors (Fig. 1H). Even if HSIL cells are mixed with other cells, they can be clearly distinguished. In this study, 13 of 45 cases of LSIL were also positive for p16. These 13 cases must be false positive or some of the lesions were in a persistent infection state in hrHPV, which will need further close follow-up in the future. In addition, the specificity of p16 for detecting HSIL and Cancer was significantly higher than that reported in other literature8. This might be because of the interpretation of the p16 results, which was completely undertaken by cytology experts with a good morphological basis.
Conclusion
In summary, the current the cytological screening of cervical cells of patients with dysplasia and carcinoma has high specificity but low sensitivity, while HPV DNA testing has improved the sensitivity. However, both techniques cannot specifically identify HSIL or worse. In the current study, p16 detection in cervical cells of patients with HSIL and Cancer may have better diagnostic performance than cytology, with sensitivity of 96.0% and accuracy of 91.7%. Our results demonstrated that p16 immunostaining in cervical brushing cells may not only be used as an ancillary tool to cytological diagnosis of cervical HSIL or worse, but also help to determine the triage of LSIL and Benign.
Footnotes
Ethical Approval: The study was conducted according to the guidelines of the institutional review boards at the First Hospital of China Medical University, and we have obtained internal review board approval.
Statement of Human and Animal Rights: Human specimens were tested in accordance with our institutional review board guidelines [2018]2018-10-2.
Statement of Informed Consent: We obtained the patients’ informed consent for this study.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China to Guang-Ping Wu, Grant No.81171650 and 81672082.
References
- 1. National Cancer Institute Workshop. The 1988 Bethesda system for reporting cervical/vaginal cytologic diagnoses. JAMA. 1989;262(7):931–934. [PubMed] [Google Scholar]
- 2. Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, McCalmont T, Nayar R, Palefsky JM, Stoler MH, Wilkinson EJ, Zaino RJ, Wilbur DC; Members of LAST Project Work Groups. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012;16(3):205–242. [DOI] [PubMed] [Google Scholar]
- 3. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed Lyon, France: IARC; 2014. [Google Scholar]
- 4. zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. [DOI] [PubMed] [Google Scholar]
- 5. Schiffman M, Wentzensen N. From human papillomavirus to cervical cancer. Obstet Gynecol. 2010;116(1):177–185. [DOI] [PubMed] [Google Scholar]
- 6. Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153(6):1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, von Knebel Doeberitz M. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92(2):276–284. [DOI] [PubMed] [Google Scholar]
- 8. Wong YP, Abdul Raub SH, Mohd Dali AZ, Kassim F, Visvalingam V, Zakaria Z, Kamaluddin MA, Noor Akmal S. P16INK4a: a potential diagnostic adjunct for prediction of high-grade cervical lesions in liquid-based cytology: with HPV testing and histological correlation. Malays J Pathol. 2016;38(2):93–101. [PubMed] [Google Scholar]
- 9. Cheah PL, Looi LM, Teoh KH, Mun KS, Nazarina AR. p16(INK4a) is a useful marker of human papillomavirus integration allowing risk stratification for cervical malignancies. Asian Pac J Cancer Prev. 2012;13(2):469–472. [DOI] [PubMed] [Google Scholar]
- 10. Khleif SN, DeGregori J, Yee CL, Otterson GA, Kaye FJ, Nevins JR, Howley PM. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclinkinase inhibitor activity. Proc Natl Acad Sci U S A. 1996;93(9):4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakao Y, Yang X, Yokoyama M, Ferenczy A, Tang SC, Pater MM, Pater A. Induction of p16 during immortalization by HPV 16 and 18 and not during malignant transformation.Br J Cancer.1997;75(10):1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang G, Yang B, Abdul-Karim FW. p16 immunohistochemistry is useful in confirming high-grade squamous intraepithelial lesions (HSIL) in women with negative HPV testing. Int J Gynecol Pathol. 2015;34(2):180–186. [DOI] [PubMed] [Google Scholar]
- 13. Shain AF, Kwok S, Folkins AK, Kong CS. Utility of p16 immunohistochemistry in evaluating negative cervical biopsies following high-risk Pap Test results. Am J Surg Pathol. 2018;42(1):69–75. [DOI] [PubMed] [Google Scholar]
- 14. Shain AF, Wilbur DC, Stoler MH, Quade BJ, Kong CS. Test characteristics of specific p16 clones in the detection of high-grade squamous intraepithelial lesions (HSIL). Int J Gynecol Pathol. 2018;37(1):82–87. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Alqatari M, Sultan K, Ye F, Gao D, Sigel K, Zhang D, Kalir T. Using p16 immunohistochemistry to classify morphologic cervical intraepithelial neoplasia 2: correlation of ambiguous staining patterns with HPV subtypes and clinical outcome. Hum Pathol. 2017;66:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miralpeix E, Genovés J, Maria Solé-Sedeño J, Mancebo G, Lloveras B, Bellosillo B, Alameda F, Carreras R. Usefulness of p16INK4a staining for managing histological high-grade squamous intraepithelial cervical lesions. Mod Pathol. 2017;30(2):304–310. [DOI] [PubMed] [Google Scholar]
- 17. Wentzensen N, Bergeron C, Cas F, Eschenbach D, Vinokurova S, von Knebel Doeberitz M. Evaluation of a nuclear score for p16INK4a-stained cervical squamous cells in liquid-based cytology samples. Cancer. 2005;105(6):461–467. [DOI] [PubMed] [Google Scholar]
- 18. Zhao C, Li Z, Nayar R, Levi AW, Winkler BA, Moriarty AT, Barkan GA, Rao J, Miller F, Fan F, Zhou Z, Si Q, Fischer AH, Sturgis CD, Jing X, Marshall CB, Witt BL, Birdsong GG, Crothers BA. Prior high-risk human papillomavirus testing and Papanicolaou test results of 70 invasive cervical carcinomas diagnosed in 2012: results of a retrospective multicenter study. Arch Pathol Lab Med. 2015;139(2):184–188. [DOI] [PubMed] [Google Scholar]
- 19. You W, Li S, Du R, Zheng J, Shen A. Epidemiological study of high-risk human papillomavirus infection in subjects with abnormal cytological findings in cervical cancer screening. Exp Ther Med. 2018;15(1):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belinson JL, Pretorius RG, Enerson C, Garcia F, Cruz EP, Belinson SE, Yeverino García E, Brainard J. The Mexican Cervical Cancer Screening Trial: self-sampling for human papillomavirus with unaided visual inspection as a secondary screen. Int J Gynecol Cancer. 2009;19(1):27–32. [DOI] [PubMed] [Google Scholar]
- 21. Solomon D, Schiffman M, Tarone R; ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001;93(4):293–299. [DOI] [PubMed] [Google Scholar]
- 22. Nayar R, Wilbur DC. The Bethesda system for reporting cervical cytology. Definitions, criteria, and explanatory notes. 3rd edition New York (NY): Springer-Verlag; 2015:175–177. [Google Scholar]